The U.S. Food and Drug Administration (FDA) is proposing a requirement to provide consumers with a front-of-package (FOP) nutrition label in packaged foods, per the agency’s official news release.
The proposal coincides with the FDA’s nutrition priorities, which are designed to help combat the U.S.’s chronic disease crisis. If finalized, the proposal would give consumers readily visible information about a food’s product’s saturated fat, sodium, and added sugars content, the agency says.
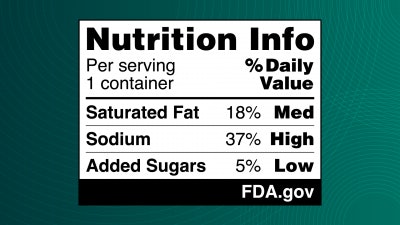
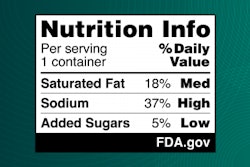
An FOP nutrition label or “Nutrition Info box” would show information on the nutrients in a simple format. The label indicates if a food contains “Low,” “Med (medium),” or “High” levels of the nutrients. Furthermore, the proposed labeling would complement the FDA’s longstanding “Nutrition Facts” label, which provides consumers more detailed nutrient content information.
FDA Commissioner Robert M. Califf, M.D. captured the impact adding the front label would have on public health.
“The science on saturated fat, sodium and added sugars is clear,” Califf states. “Nearly everyone knows or cares for someone with a chronic disease that is due, in part, to the food we eat. It is time we make it easier for consumers to glance, grab, and go. Adding front-of-package nutrition labeling to most packaged foods would do that. We are fully committed to pulling all the levers available to the FDA to make nutrition information readily accessible as part of our efforts to promote public health.”
The FDA claims the proposed Nutrition Info box is informed by a substantial body of research, which includes scientific literature review, consumer focus groups, and peer review.
Should the proposal go through, food manufacturers would be required to add the front label to most packaged foods three years after the final rule’s effective date for businesses with $10 million or more annual food sales. For businesses with less than $10 million in annual food sales, the label would need to be added four years after the rule’s effective date.