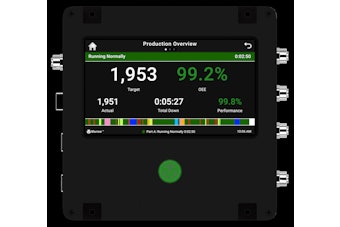
The approved claim states: “Supportive but not conclusive research shows that consumption of EPA and DHA omega-3 fatty acids may reduce the risk of coronary heart disease.”
The label must list the number of grams of DHA and EPA in a single serving. Except for fish and dietary supplements, foods containing the qualified claim also must be low in cholesterol, low in saturated fat, and have less than 15% of calories derived from saturated fat. FDA recommended that consumption of EPA and DHA not exceed 3 g per day. No more than 2 g should come from dietary supplements.