The U.S. Food and Drug Administration on Feb. 14 reported that a counterfeit version of Genentech’s (a member company of Roche) cancer treatment medication Avastin was in U.S. distribution.
Roche and Genentech noted that they are “implementing special packaging and printing techniques that make counterfeits both more difficult to make and easier to spot,” according to a press statement on Genentech’s Web site.
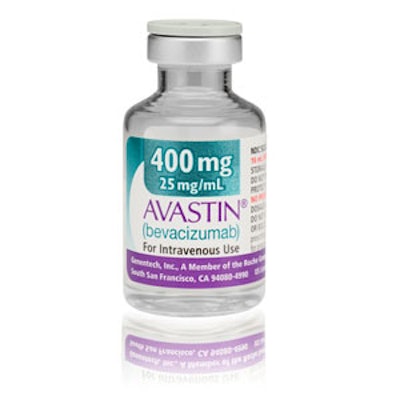
Genentech’s release added, “The counterfeit product is not safe or effective and should not be used. Chemical analyses of the counterfeit vials tested to date have confirmed the product does not contain the active ingredients for Avastin. Patient safety is Roche and Genentech's primary concern. We are working with the U.S. Food and Drug Administration and law enforcement to aid their evaluations, determine the source of the counterfeit drug, andrevent its further distribution.” (An authentic version is shown here.)
FDA said it “is warning health care professionals and patients about a counterfeit version of Avastin 400mg/16mL, which may have been purchased and used by some medical practices in the United States. Avastin is an injectable medicine used to treat cancer and is administered to patients in clinics, hospitals, and doctors’ offices. The counterfeit version of Avastin does not contain the medicine’s active ingredient, bevacizumab, which may have resulted in patients not receiving needed therapy.
“In a related action, FDA has issued letters to 19 medical practices in the United States that purchased unapproved cancer medicines that may include the counterfeit Avastin. The counterfeit version is labeled as Avastin, manufactured by Roche. Roche is the company that manufactures Avastin approved for marketing outside of the United States.
“Roche conducted laboratory tests that confirmed the counterfeit version of Avastin. Packages or vials may be counterfeit if they are labeled with Roche as the manufacturer, display batch numbers that start with B6010, B6011 or B86017.”
The agency noted, “The only FDA-approved version of Avastin for use in the United States is marketed by Genentech (a member company of Roche). The FDA-approved version does not include the Roche logo on the packaging or vials. In addition, Genentech’s FDA-approved version of Avastin vials and packaging have a 6-digit numeric batch number and expiration dates in a 3-letter month and 4-digit year format (e.g., JAN 2014). Genentech’s Avastin products are safe and effective for their intended uses.”
FDA said, “The 19 medical practices in the United States purchased unapproved cancer medicines and, potentially, the counterfeit Avastin, from Quality Specialty Products (QSP), a foreign supplier that may also be known as Montana Health Care Solutions. Volunteer Distribution in Gainesboro, Tennessee is a distributor of QSP’s products. FDA has requested that the medical practices stop using any remaining products from these suppliers. FDA cannot ensure the safety or efficacy of any of these unapproved products.”
Genentech’s Web site showed images of the authentic and counterfeit Avastin, noting, “the following is true for all authentic Avastin that is FDA-approved for use in the United States:
• All cartons and vials approved for use in the United States have "Genentech" or "Genentech, a member of the Roche Group" printed on the labels;
• The lot number on the carton and vial should be 6 digits with no letters;
• The expiry date is formatted as a 3-letter month and 4-digit year, e.g. JUL 2014;
• The date of manufacture is not printed on the carton or vial;
• All the text on the vial labels, cartons, and package inserts is English.”
Both Web sites provide additional Web links and resources that include information about counterfeit meds, as well as for consumers and/or healthcare providers.

























