A two-year-old boy is rushed to a Kwale hospital in Kenya’s Coast Province. The clinical officer at the health facility diagnoses febrile convulsions and quickly rushes to the hospital’s pharmacy for a dose of medicine that will ease the boy’s suffering. But nothing happens, so the boy is given additional doses but still the boy does not respond. Finally, he dies. Only later, upon investigation, is it discovered that the drug had no active ingredients. The drug was a fake.
Counterfeit drugs in Kenya have been known to exist for a long time. Walk into any pharmacy in search of a certain drug prescribed by a doctor. If the price is too high, the pharmacist will tell you of yet another prescription drug that works just the same but comes cheaper than the one requested.
“There is no difference whatsoever,” he is quick to say, “the prescription is the same, the difference is just the manufacturing company,” he adds as he quickly wraps the cheaper drug.
The magnitude of the sale of counterfeit drugs was felt several months ago when a popular anti-malarial drug was discovered to be a fake. Officials from the Pharmacy and Poisons Board, a government watchdog that registers pharmaceutical firms and pharmacies, discovered thousands of fake anti-malarial drugs stashed in a downtown Nairobi electrical shop.
Shocked by the discovery, Beijing Holley Cotec, the Chinese company that manufactures the popular artemisinin-based drugs Cotecxin and Duo-Cotecxin, immediately recalled all the drugs in the Kenyan market for analysis. The firm also elected to re-introduce the popular drugs, this time under strict packaging guidelines that would make it harder for counterfeiters to duplicate.
Duo-Cotecxin is one of the artemisinin-based combination therapy drugs that is highly recommended by the World Health Organization (WHO) in the treatment of malaria. The drug is widely supplied in government and private hospitals in Kenya.
According to the Kenya government’s chief pharmacist Dr. Fred Siyoi, a full dose of Duo-Cotecxin costs about Kenya Shillings (Kes) 350 (US$5) in Kenya while the counterfeited drug was selling for less than Kes 70 (US$1) in Nairobi and elsewhere in the country.
A counterfeiting first
Speaking at a press briefing, the head of the Kenya government’s anti-malarial control unit, Dr. Willy Akwale, said this is the first case of a counterfeit supply of artemisinin combination therapy drugs. Said Akwale: “There have been many counterfeits on the sulphur-based anti-malaria drugs before, forcing us to have difficulties in countering the disease.”
Kenya’s Director of Medical Services Dr. James Nyikal says the Ministry of Health is committed to ensuring that all drugs on sale in the country are safe and meet the established standards of quality and that inspection of fake drugs has been intensified across the country.
He says the Pharmacy and Poisons Board has set up an elaborate system for evaluating quality of drugs before and after registration for sale in Kenya.
According to Dr Wilfred Ochieng, head of Pharmaceutical Inspectorate, the Pharmacy and Poisons Board regularly samples medicines on sale in the market and analyzes them at the National Quality Control Laboratories and at the University of Nairobi to ensure that they adhere to established quality standards. He says the board has posted drug inspectors at all ports of entry to control entry of medicines into the country.
Quality analysis done by the Pharmacy and Poisons Board on the discovered counterfeit anti-malarial drugs showed that they did not contain the active anti-malarial ingredient and cannot therefore be used in the treatment of malaria. The board immediately issued a public alert in the local press and gave guidelines on how one could tell the fakes from the genuine drugs.
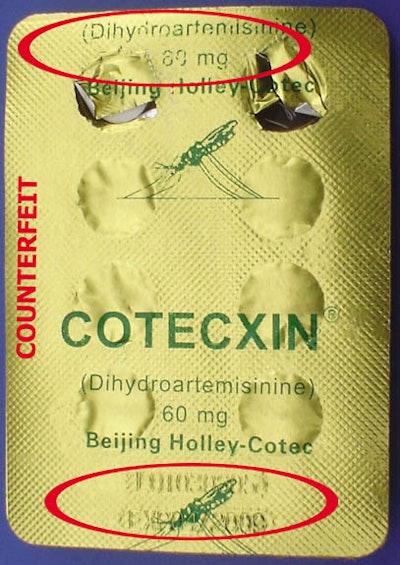
Says Dr. Siyoi: “From outside, the counterfeit pack looks similar to the original product and may be difficult to tell apart. Some packs even bear a batch number similar to one of the genuine batches officially supplied last year.”
Contrary to what is stated on the label, notes Dr. Siyoi, the counterfeit products have not been manufactured in the facilities of Beijing Holley-Cotec Pharmaceuticals, the sole license holders and manufacturers of Duo-Cotecxin and Cotecxin. He says there are differences between the packaging containing the genuine and counterfeit drug. The difference is on the blister pack, where the name of the manufacturer is indicated on the genuine pack but not on the pack holding the counterfeit.
Another difference that Dr. Siyoi identifies is that the printing on the blister pack containing the counterfeit drugs fades easily on rubbing with a moist finger. On a package of the genuine drug, this does not happen.
Investigation is ongoing
Dr. Nyikal says investigations and inspections are ongoing to determine the extent of the distribution of the counterfeit Duo-Cotecxin and Cotecxin drugs. The goal is to identify individuals or companies behind the sale of the counterfeit drugs. In a recent interview with the press, Eric Law, Beijing Holley-Cotec Pharmaceutical’s vice president, says that they have yet to locate the source of the counterfeits, but there is strong evidence linking the source to Asia.
“We are going to introduce a new technology to bring tamper-evidence to doses that will be supplied to replace the withdrawn drugs,” Mr. Law told the press.
The Chinese drug manufacturer recalled more than 20,000 doses of the anti-malarial drugs from the Kenyan market and will replace them with new drugs in new tamper-proof packaging that has a three-dimensional hologram seal on the outer pack. “Make sure you find this hologram on the packaging,” Law advises. If the packaging does not come with the hologram, he says, then the drugs are not genuine. According to Law, the new packaging will make it difficult for the cheats to replicate and sell fake drugs into the Kenyan market, where more than 35,000 people die of Malaria each year.
Martin Kimani, the Kenya sales executive at Beijing Holley-Cotec, says the discovery of the fake drugs in Nairobi was a great eye-opener for the firm, which will now constantly check on its products put in the marketplace.
In a telephone interview, Kimani indicates that a packaging security solution in addition to the three-dimensional seal has also been deployed. But he would not divulge details. Neither would he give details on the losses the firm has incurred following the sale of the counterfeit drugs.
“With the new security packaging we have introduced, we are a few steps ahead of the counterfeiters,” he says.
Kenyan health officials warn of a global health catastrophe if the growing trade in fake anti-malarial drugs leads to widespread resistance. Making matters worse, sophisticated trans-national gangs are thought to be behind the counterfeit drugs, a fast growing multibillion-dollar business.
Counterfeiters have made a killing in Kenya’s war on Malaria and have also greatly benefited from the booming sale of erectile dysfunction (ED) drugs as well. In the summer of 2007, it was discovered that the popular drug Çialis was a hot sell. Counterfeiters jumped at the opportunity, much to the amazement of the Pharmacy and Poisons Board. Çialis’ manufacturer Eli Lilly says the counterfeiters in the local market have reached levels that have drastically undermined their trade in the country, forcing the pharmaceutical firm to adopt a new security packaging system.
“Barely six months after the launch of the drug in Kenya, illegally imported Çialis had eroded 70 percent of our market,” says Steve Mburu, the firm’s marketing manager. “When we check with doctors and pharmacists, the sales are ever soaring, but this is never reflected in our books. It is not until doctors complained that our product was no longer producing the expected kick that we took a closer look. We saw a lot of fakes, counterfeits, and even some dangerous products masquerading as Çialis.”
New security pack
To fight the counterfeiters, the manufacturer recently introduced a security pack with four distinct features that include an oval sticker that looks different when the pack is viewed from different angles. Other security packaging measures put in place include tamper-evident seals and color-shifting ink logos.
Recently, the National Quality Control Laboratories (NQCL) and the Pharmacy and Poisons Board carried out a random survey of drugs in the Kenyan market and found that almost 30 percent of drugs are counterfeit. Says Dr. Hezekiah Chepkwony, the director of the NQCL: “Some of the drugs are no more than just chalk or water being marketed as competent pharmaceutical products.” The fake drugs, notes Dr. Chepkwony, range from dangerous products to totally ineffective ones.
Trade in counterfeit drugs is a lucrative business and the figures speak for themselves. According to figures from the Kenya Association of Pharmaceutical Industry, counterfeit pharmaceutical products account for approximately $130 million annually in sales in the country.
“Counterfeiting is a plague in Africa; it is a crime to sell fake drugs and governments need to consider increasing penalties against the distribution and selling of counterfeit drugs,” says Karl Lintel, Pfizer regional director.
Pfizer, says Lintel, has set up a department to deal with counterfeit drugs. The department is mandated to conduct regular check-ups in the market, and the company shares information with the relevant authorities so that the public is warned in time.
Many experts blame the country’s legal systems for not being serious in fighting counterfeits.
“The penalties for such a criminal act are in most cases inadequate,” notes Dr. William Mwatu, the medical and regulatory affairs director at East Africa GlaxoSmithKline. “We are currently seeing an increase in the hawking of medicines from pharmacy to pharmacy by unauthorized persons. We need to ensure that we sell drugs to authorized persons and buy only from authorized persons, if we hope to stop counterfeits,” Mwatu explains.
Denis Gathanju is a freelance writer based in Kenya, Africa.