So much of the food and beverage industry’s safety and quality assurance operations are still manual, but many of the most efficient companies are turning to electronic systems to eliminate errors and increase efficiencies. But even electronic systems can be inefficient when different systems are used for different parts of the food safety plan—but not the whole plan. Other times, these systems don’t “talk” to each other.
Preparing your plant for compliance with the Food Safety Modernization Act (FSMA) and an array of multiple regulations and standards can seem an insurmountable challenge in at least four ways:
The sheer volume of paper. Gathering and maintaining all of the documents and records that verify and validate the various components of food safety plans can be extremely time-consuming and costly, especially when it takes workers’ time away from other duties. The more types of audits, the more complex it becomes to gather records completely, accurately, and in a timely manner.
Proof that plans are being carried out correctly. In addition to gathering records, there’s also the challenge of making sure everything is in conformance with requirements.
Preparing for an audit is not the time to find out that one facility is using old forms, or a manager wasn’t aware of a new or modified Critical Limit or Preventive Control for a given piece of your process.
Supplier/vendor management. Whether it’s for FSMA’s Foreign Supplier Verification Program (FSVP), GFSI’s Approved Vendor Programs, customer requirements, or your own food safety plans, it’s a huge challenge to track all of the necessary specifications, registrations, supplier audit documents, and proof of hazard analysis/Preventive Controls.
Response time. The time associated with meeting the above three challenges can be excessive—and that’s for the audits you know about. When it comes to unannounced audits from customers, the FDA, SQF, or other standards bodies, the word “excessive” can be replaced with “disruptive” or worse.
For such reasons, demand has risen for dedicated management software systems that ensure that companies will comply with food quality and safety regulations.
What is an automated food quality/safety system?
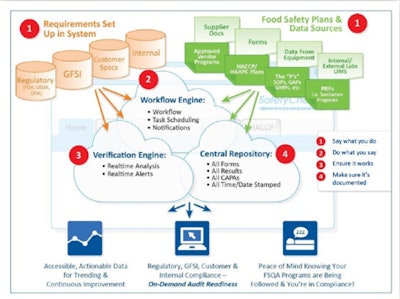
An automated food quality and safety management system integrates real-time data collection and analysis with specifications—along with automatic alerts when deviations are detected—to prevent non-compliant ingredients and raw materials from coming into a facility and non-conforming finished products from going out. It also helps ensure related workflow, processes, and documentation, and does so in a central repository of quality and safety data for trending, assessments, reporting, and of course, audit readiness.
Systems use various types of information technology infrastructure, but all provide an integrated repository for all data; requirements of various kinds of audits; food safety plans and data sources; and provisions for workflow, data storage, and verification. (See diagram on this page.)
Implementing a dedicated software system can allow companies to eliminate problems such as wasting time entering instead of analyzing data; searching hundreds of supplier documents to find the one or two that require action; incomplete revision logs; falling behind in facility inspection corrective actions; and discovering missing or mis-filed information only when found in an audit—which may be announced or unannounced.
Depending on your company’s compliance requirements, you may need to manage programs that call for up to four types of audits, each with unique characteristics:
Regulatory audits. USDA requires proof of Hazard Analysis & Critical Control Points (HACCP) pre-shipment reviews before products are put into commerce, and can ask to look at documents for every single day of operation versus a time period. The FDA can arrive unannounced if they receive a complaint, and requires answers to most queries in two hours.
GFSI audits. GFSI audits require full documentation of compliance with approved vendor programs as well as documentation of PRPs, such as preventive maintenance and food defense. And, it was recently announced that, going forward, SQF will require one in every three audits to be unannounced.
Customer audits. In addition to safety documentation, customer audits also focus on compliance with your customers’ specific quality specifications—weight, moisture, packaging, and the like.
Internal audits. These can be the most difficult, because they can encompass requirements from all of the other types of audits combined. They may also require operational documentation on Key Performance Indicators (KPIs). Additionally, FSMA’s FSVP and proposed third-party auditor rules, which will require food safety violations to be reported directly to the FDA, could be a game changer when it comes to internal audits.
Common elements of all types of audits:
-
You must show that you say what you are going to do, so your food safety plans, risk assessments, Preventive Controls-related SOPs, CCPs, PRPs, GMPs, etc., must be well defined, organized, and accessible.
-
You must show that you did what you said, and therefore you must be able to verify scheduling and completion of tasks, and ensure that test results become part of your records.
-
You must prove through analysis and scientific validation that what you’re doing is working. Therefore you must validate that the frequency of your inspections is correct, and that your critical limits are working.
-
You have to show that you analyzed information for CAPAs. The required corrective and preventive actions (CAPAs) must be completed, and management must get this information in a timely fashion.
-
You must show continuous improvement. In meeting this need, you must consider questions such as: Is there new scientific information? New regulatory or GFSI requirements? Is your level of corporate food safety and quality assurance able to leverage best practices from the plant level?
-
You must have accurate, audit-ready documentation for all of the above considerations. All responsible food safety and quality organizations are doing these things. But to an auditor, if an activity isn’t properly documented, it’s as if you never did it.
Benefits of automated ‘readiness’
With the right integrated food quality and safety management software system, food safety plans, customer and internal quality specifications, and approved vendor programs are carried out on time, according to plan, and with the efficiency of a single, integrated system. Tasks happen when they are supposed to, and issues are dealt with in a timely, preventive manner. This is the first step in preparing for any audit.
Because every task, document, record, test result, and action is time/date-stamped, the system provides unalterable records for greater audit efficacy. The robust nature of data recording also supports continuous improvement, something called for in almost all audit schemes and best practices.
Having a central repository helps organize and simplify reporting of all forms of audit documentation as well as reporting against internal KPIs. When the implementation uses current-generation technology, the value of this data is enhanced further through remote access to data using secure “cloud” or Internet-based sharing for greater transparency and visibility across plants, companies, and supply chain partners.
This article adapted and edited based on information from SafetyChain Software.
Liked this article? Download the entire Food Safety Playbook here.
















![Xl Touch Scoreboard Screen High Res[39]](https://img.packworld.com/mindful/pmmi/workspaces/default/uploads/2025/12/xl-touch-scoreboard-screen-highres39.JHTycZQYnN.png?auto=format%2Ccompress&fit=crop&h=227&q=70&w=340)







