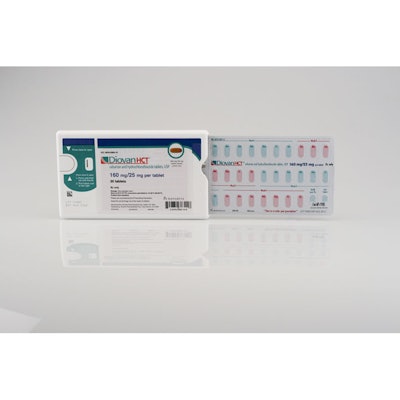
Novartis Pharmaceuticals’ Diovan HCT® Shellpak® was named 2010 Compliance Package of the Year at the Healthcare Compliance Packaging Council’s (HCPC) annual Compliance Package of the Year (CPY) competition May 3 at RxAdherence 2011, the organization’s annual conference on patient adherence and compliance-prompting packaging issues.
The competition consists of two categories: one for trade packages released in the past year and the other for innovative designs not yet used commercially. Winning for Innovative Design was the DelPouch® Starter Kit by Catalent Pharma Solutions. The CPY First Runner Up was awarded to the Somaxon® Pharmaceuticals’ Silenor® Patient Starter Kit, while CPY Second Runner Up was earned by Three Rivers Pharmaceutical’s Ribasphere® Ribapak for Ribavirin tablets.
Submitted by Anderson Packaging on behalf of Novartis Pharmaceuticals, the Diovan HCT Shellpak (shown) features 30 days of treatment in a calendarized unit-dose blister. To facilitate compliance with the medication regimen, tablets are laid out with color-coded days and weeks, including reminders for refilling the prescription.
The 30-day blister is contained in the 170-mm Shellpak outer, patented child-resistant package design from MeadWestvaco. The rigid plastic design features a front and back label. The back label provides a designated area for the patient’s prescription label as well as an adhered prescription insert. The front of the pack features an extended-content booklet label, including a photograph of the pill. Multiple pages within the front label provide patients assistance with dosing instructions, guides to joining the BP Success Zone Program including the Web site, toll-free phone number, and additional regulatory information.
The Diovan HCT Shellpak is offered in four strength combinations. Each strength combination features a distinctive color (brown, blue, purple, red) and photograph of the unique tablet design for each strength to ensure correct dosing for the patient.
Innovative starter kits
The DelPouch Starter Kit from Catalent helps to drive patient adherence. Designed to enhance the patient’s engagement and experience, the package is tailored to provide convenient, simplified dosing to improve adherence of topical products. The kit incorporates multiple adherence drivers, such as the DelPouch unit-dose delivery system, the dynamic connectivity of Catalent’s Media Enhanced Packaging™ technology, and reminder-prompting configurations.
Also submitted by Anderson Packaging, on behalf of Somaxon Pharmaceuticals, the Silenor Patient Starter Kit’s carton is designed so that when opened resembles a bedroom complete with bed and nightstand. Contained within the design is a seven-count unit-dose carded blister, removable from the design to enable portability and convenience.
To support patient compliance and adherence, the bed carton design contains a literature pocket, housing the medication guide, the Sleep-Saver™ Program prescription discount card, and a multi-panel color leaflet complete with instructions on taking the product, description of side effects, guides to enrolling in the Sleep-Saver program including Web site and toll-free phone number, as well as additional information on insomnia treatment.
Meanwhile, Three Rivers Pharmaceutical’s Ribasphere Ribapak for Ribavirin Tablets. RIBASPHERE is indicated for the treatment of adults with chronic hepatitis C virus infection. The product was packaged in Three Rivers’ Ribapak by Sharp Corp.
The Compliance Package of the Year winner, the First Runner-up, and the Innovative Design winner will be asked to designate scholarship funds to a university-level packaging school focused on the pharmaceutical industry. Complete competition guidelines are available on HCPC's Web site or by phone at 804-338-5778.

























