The Comar DoseGuard™ Valved Bottle Adapter System is receiving public recognition as the dosing device with the preferred safety feature for protecting children from accidental overdose of liquid medications, such as acetaminophen. Recent consumer testing showed the closed flow restrictor valve used in the DoseGuard system was more effective than other devices in preventing young children from extracting the contents of a medicine bottle in an unsupervised setting.
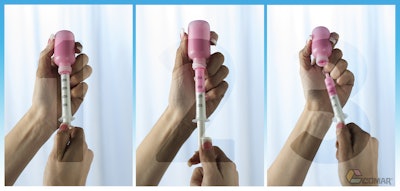
The DoseGuard system consists of a bottle, bottle adapter with a closed flow restrictor valve, and an oral syringe, all designed to work together as a complete system. The bottle adapter valve remains closed tightly until the tip of the syringe is inserted into the valve opening. A snap alerts users that the tip is seated and the valve is open. Users invert the bottle and pull back on the syringe to withdraw the correct dose. When the syringe is removed the valve closes and reseals the bottle.
Tests show the valve reseals after repeated openings. Because the bottle adapter is securely attached in the neck of the bottle, the possibility of it being removed or pressed through the bottleneck is virtually eliminated, adding another unique level of safety to the packaging system.
Accidental overdoses are increasing for children 6-years old and younger. A recent study reported that more than 60,000 young children are rushed to emergency rooms for unintentional overdoses annually. Medications now exceed household products (e.g., cleaning fluids) as the primary cause of pediatric poisoning. Regulatory action is imminent. To develop preventive strategies, the U.S. Centers for Disease Control and Prevention (CDC) formed the PROTECT Initiative, bringing healthcare professionals together with leading over-the-counter medication and packaging manufacturers. Comar is a member of the initiative.
The Comar DoseGuard system is protected by patents. Comar manufacturing facilities comply with current Good Manufacturing Practices (cGMP) and ISO 9001:13485 quality standards. For additional information or samples, call: 1-856-507-5409 or email [email protected].