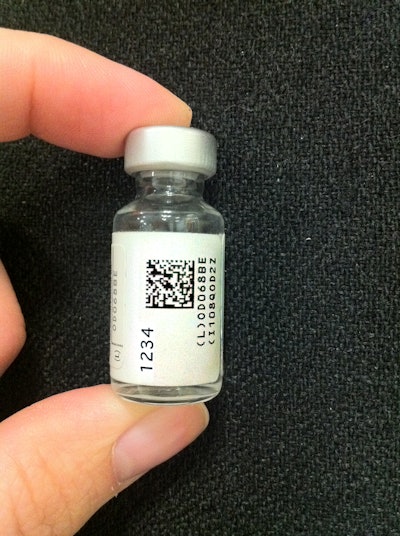
The American Academy of Pediatrics (AAP) and Sanofi Pasteur, the vaccines division of Sanofi, recently announced that pediatric offices across the country will now have access to a new vaccine packaging technology that will capture more product information in a simple two-dimensional (2D) scan--including lot numbers and expiration dates--than was possible in a standard linear bar-code scan.
Launched Dec. 2011, the new technology is designed to reduce medical errors and help health care providers document vaccine information in patient records with greater accuracy.
"The 2D bar-code technology is available because of the work by a collaborative group of stakeholders and the forward thinking of many AAP members," says Edward Zissman, MD, FAAP, co-chair of the AAP Vaccine Barcoding project. "Through the use of this technology, healthcare professionals can enhance the level of care they provide, increase office efficiency, and better manage product inventory."
The technology will first be introduced on two Sanofi Pasteur vaccines--pediatric Diphtheria and Tetanus Toxoid Adsorbed (DT) vaccine, and Menactra(r) Meningococcal (Groups A, C, Y, and W-135) Polysaccharide Diphtheria Toxoid Conjugate vaccine. Other vaccine manufacturers are expected to begin launching products with 2D bar codes later in the year.
"There is a clear need for a system to record product information with a higher level of speed and accuracy, and we aim to address this need with the 2D bar-code technology on our vaccines," says Chad Hoover, vice president, Chief Commercial Officer, Sanofi Pasteur U.S. "As an industry leader and healthcare partner, Sanofi Pasteur is pleased to be the first vaccine manufacturer to bring this innovative technology to providers offices."
Sanofi Pasteur will institute a tiered approach to the 2D bar-code product introduction to ensure supply is not affected by the new packaging technology. The AAP has developed a clinical guidance for physicians to help practices use 2D bar coding with their electronic medical record (EMR) or state immunization information system (registry).
www.aap.org/immunization.
In Sept. 2012, the Centers for Disease Control and Prevention will launch an eight-month pilot project designed to assess the challenges and determine best practices for labeling and tracking vaccines using 2D bar codes.
The pilot will test the implementation of 2D bar codes on selected vaccines, and evaluate and document the impact of 2D bar coding on manufacturers, immunizers, and reporting systems. Participants in the pilot will receive scanning devices, software, and training. The pilot also will address implementation opportunities with electronic medical records and state immunization information systems.
The project is enrolling one to three manufacturers, 10 states/grantees and 300 public, private, and commercial immunizers. Those interested in participating should e-mail [email protected]. The team is looking for participants from Alaska, Florida, Michigan, New Jersey, New Mexico, New York, New York City, Ohio, Oregon and Washington, who will report to their respective state/grantee registries.
The enrollment process for manufacturer, grantee, and immunizer candidates is expected to be completed by February, after which time equipment will be installed and participants will be trained. The AAP is a partner on this project and will share information as it becomes available.
The American Academy of Pediatrics is an organization of 60,000 primary care pediatricians, pediatric medical subspecialists, and pediatric surgical specialists dedicated to the health, safety, and well-being of infants, children, adolescents and young adults.

























