The Healthcare Compliance Packaging Council awarded the winners of its annual Compliance Package of the Year competition at RxAdherence 2014, the organization’s annual conference on patient adherence and compliance-prompting packaging issues.
The annual competition recognizes the most innovative pharmaceutical packages designed to improve patient adherence. This year’s winners are:
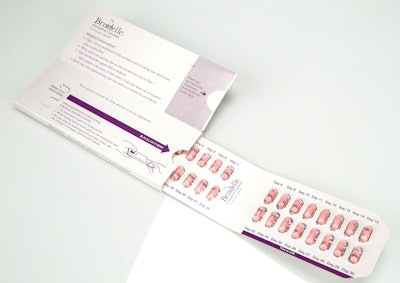
• 2013 Compliance Package of the Year: Noven Therapeutics Brisdelle in MWV Dosepak® from PCI (Packaging Coordinators, Inc.)
• Compliance Package of the Year First Runner Up: Depomed® Gralise® Starter Pack from PCI
• Compliance Package of the Year Second Runner Up: Mylan Amlodipine Besylate in MWV Shellpak® Renew with Optilock® technology
• 2013 Innovative Design Winner: Mem-O-Ring Medication Reminder Ring for Self-Administered Opthalmics
Package of the year
Noven Therapeutics’ Brisdelle is indicated for the treatment of moderate to severe vasomotor symptoms (VMS) associated with menopause. The Brisdelle compliance design features calendarized dosing for 30 days of therapy, with a designated area for the patient to note the starting date of the therapy.
The Brisdelle package graphics highlight that the patient should take one capsule each day at bedtime. The calendarization of the product also highlights the need to refill the prescription in the final week of the packaged product. Package graphics highlight the push-and-pull mechanism for accessing the child-resistant protection for the package. The Prescribing Information and Medication Guide are conveniently located in the fifth panel of the package.
Many of the judges commented on the combination of several compliance features together in one package: calendar scheduling; clearly identified medication guide; child resistance; and product branding. One judge noted that the package “features great messaging to the patient on the billboard space, offering supportive, reassuring, friendly language. Packaging use cues are sufficient and effective, and the insert is folded appropriately for the package dimensions. The ability to write in the regimen start date and the numbered doses will help patients keep track of dosing, avoiding some of the confusion that can arise when designs incorporate the days of the week and patients begin therapy midweek.” Most judges agreed that the pack represented “a well thought-out design.”
First runner-up pack
Gralise is indicated for 24-hour pain control for patients with after-shingles pain, also known as postheraputic neuralgia or PHN. The Gralise Starter Pack is designed as a titration therapy, bringing patients from an initial dose of 300 mg on day one, progressing to the maintenance dose of 1800 mg by day 30. Patients take a single 300-mg tablet on day one, a 600-mg tablet on day two, a combination of 300 mg and 600 mg for days three through six, two 600-mg tablets and a single 300-mg tablet on days 11 through 14, and three 600-mg tablets for days 15 through 30.
The pack features extensive dosing instructions to help guide the patient through the dosing schedule. Patients are also encouraged to take Gralise with food, clearly identified on the pack labeling along with the opportunity to write the start date of the medication. The multipanel wallet-style card also includes a medication guide located in a convenient pocket. The corresponding prescribing insert is adhered to the back of the folded package.
The Gralise package was only two points below the Noven Therapeutics Brisdelle in the final summation of the judges’ evaluations. One judge commented that the “Open Here” instruction typeface could have been larger with an arrow, but then stated, “once opened, however, the panels open up almost magically. It’s a joy to use, with print and color used nicely to provide usage instructions. Really like this approach from a creative design perspective and can see how this would have a positive impact on patient compliance/adherence.”
Other judges commented, “An impressive starter package design incorporating 78 large tablets that nest very well together. The panels also feature supportive patient language and answer basic questions and provide a sturdy pocket for storing the medication guide.”
“This package addresses the unusual challenge of providing 78 pills of two different dosages to be taken by patients over a 30-day period. It is laid out with very clear instructions on what to do each day. Successful compliance should be higher as a result.”
Second runner-up package
Shellpak Renew with Optilock® technology reportedly is the smallest F=1 child-resistant adherence package on the market. It is designed to meet patient and pharmacist needs by enabling greater patient portability and improving pharmacy efficiency. The package features an advanced locking mechanism for the highest level of child resistance (F=1) in a significantly reduced package size. It is 23% smaller than the original Shellpak Renew, while still accommodating the same
medications.
Shellpak Renew with Optilock technology features a calendared blister and space for patient educational information, which is shown to improve patient adherence to medications. The decreased package size provides a more convenient way for patients to access and carry their medications, while also making dispensing medications quicker and easier for pharmacists.
Mylan, one of the world’s leading generics and specialty pharmaceutical companies, was believed to be the first to package product in the new Shellpak Renew with Optilock technology, beginning in the summer of 2013. MWV’s packaging is aligned with Mylan’s mission to meet unmet needs by making prescription adherence more convenient for patients.
Shellpak Renew with Optilock technology was designed using insights from both customers and consumers, and every element is optimized to be space well spent. The package’s outer carton is made of MWV’s Natralock®, paperboard-based packaging that maximizes the use of recyclable materials and minimizes waste. The secure, tear-resistant carton does not require paper backing on the foil blister, making pill dispensing easy. In addition, the package contains enhanced adherence features, including a smooth-slide blister for easy medication access and color-coded spine labels to help distinguish among medications.
One judge commented on the Mylan package, “Admirable for its convenience and user-friendliness, this pack offers simple one-two instructions to open the pack and pull out the medication card. At that point the blister clearly indicates day of the week instructions for a four-week period, with a handy reminder for order refills. This pack prompts patient compliance and is quite portable. This version of the package for Mylan is considerably easier to open than previous versions of the package style.”
Innovative design winner
The Mem.O.Ring is a visual reminder that takes the guesswork out of “when” and “whether” medication was taken. Medication adherence tools for eyedrops are limited. There is no daily “Pill Minder” for drops. Multiple medication regimens for post-surgery or chronic conditions can be confusing or overwhelming for patients wanting the best medical outcomes.
The patent-pending Mem.O.Ring represents a 24-hour cycle, which is reset each day to the first dosing time. Medication name is clearly identified on the top band. Color bands correlate with cap color to simplify drug identity. Dosing schedule is boldly identified with time, notated text, or pictograms. A die-cut sleeve option may be positioned between the medication container and Ring to serve as indicator for next dosing and securing portability.
Mem.O.Ring offers benefit to pharmaceutical manufacturers as well. Medication name and manufacturer logo are clearly identified and reinforce patient association with improved outcome for long-term customer loyalty. In addition to ophthalmic, the innovation has application for optic, nasal spray, inhaler, and oral medications.
While some judges provided helpful critiques that can be used to further develop the design, others found the innovative design “an interesting idea for ophthalmic products.”
The Compliance Package of the Year winner, the First Runner-up, and the Innovative Design winner will be asked to designate HCPC-donated scholarship funds to a university-level packaging school focused on the pharmaceutical industry.
To see a spin + zoom 360° photo, click here.