By now you’ve probably seen the TV commercials on print/online ads showing the animated Cologuard box character demonstrating how Exact Sciences’ colon cancer screening test kit is as easy to use as “get, go, gone.”
Exact Sciences markets Cologuard colon cancer screening test kits directly to consumer-patients with the goal of early detection by encouraging patients to ask their doctors to prescribe the test. Cologuard is designed for patients aged 50 or older and is available by prescription only.
Once a doctor prescribes Cologuard for a patient, Madison, Wis.-based molecular sciences company Exact Sciences receives an order for the test. Automatically, Exact Sciences’ IT system, which is connected with UPS, sends a request to UPS’ Louisville warehouse to ship the kit to that patient. All Cologuard tests are housed in UPS’ Louisville facility, as UPS is the sole carrier for the product.
All shipments must arrive to the patient, anywhere within the continental U.S., within 72 hours of the order receipt. For patients, that’s the “get” part. This quick turnaround helps ensure that patients comply with taking the test by providing a stool sample, the “go” part, and returning the kit, the “gone” part.
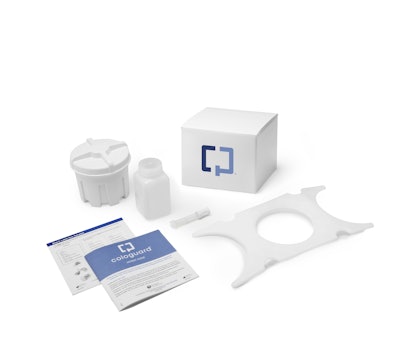
UPS facilitates the labeling of the collection kits and includes two pressure-sensitive labels, one for delivery to the patient and a second for return to Exact Sciences’ 30,000 square-foot Madison lab/R&D facility. Thermal-transfer paper is used for the label materials, which are printed flexographically. The outer kit carton is a 12-pt SBS board offset-printed in three colors plus coating.
When the patient is ready to return the kit, they can either call UPS to schedule a pickup, call Exact Sciences to schedule a pickup for them, or drop their kit off at any UPS store. Patients overnight mail the sample kit to Exact Sciences’ laboratory via either UPS’ Louisville facility or a Middleton, Wis. UPS distribution center. Cologuard test results are delivered to the ordering physician within two weeks of Exact Sciences’ lab receiving a patient’s sample.
Exact Sciences examines the sample for the presence of 11 molecular markers that may indicate the presence of colorectal cancer or advanced adenomas. A positive, or negative, result is determined, with results sent to health care providers. Patients with positive results are advised to undergo a colonoscopy. More than 25,000 physicians now offer Cologuard to their patients, and more than 100,000 patients have completed the test.
FDA regards test as a first
When the FDA approved Cologuard in 2014, the agency called it, “the first non-invasive DNA screening test for colorectal cancer.” Following 20 years of research by Exact Sciences, the announcement was big news.
“This is the first time in history that FDA has approved a technology and CMS [Centers for Medicare & Medicaid Services] has proposed national coverage on the same day,” said Patrick Conway, Chief Medical Officer and Deputy Administrator for innovation and quality for CMS. “This parallel review represents unprecedented collaboration between the two agencies and industry and most importantly will provide timely access for Medicare beneficiaries to an innovative screening test to help in the early detection of colorectal cancer.”
Packaging a ‘game-changer’
Tammy Turek-Etienne, PhD and Vice President of Operations at Exact Sciences, says Cologuard is regulated as a medical device in the in-vitro diagnostics therapeutic category; as a screening test. It has no pharmaceutical or treatment properties.
She notes, “In addition to groundbreaking science, what also sets Cologuard apart is that Exact Sciences designed the test in a way that makes the collection kit, as well as the process of taking it, as patient- and physician-friendly as possible. A key component of this ease-of-use is the packaging.
“Each Cologuard kit contains a shipping box filled with a plastic sample container, plastic bracket, patient instruction guide, sample labels, a bottle of preservative liquid and a tube containing a buffered detergent solution with an antimicrobial agent,” says Turek-Etienne.
Exact Sciences worked with an outside consultant to design the collection kit with the user experience in mind and for the specific demographic the product would be used by—those ages 50 to 75.
“Taking into account research about user experiences with other at-home screening tests, issues and limitations this population may have in their ability to physically open and correctly close the container, as well as extensive human factors testing, Exact Sciences designed a collection kit specifically for this age demographic,” says Turek-Etienne.
Packaging is outsourced
The packaging function is outsourced to Phillips-Medisize in New Richmond, Wis., where a single packaging line is utilized to assemble the Cologuard Collection Kits.This line has the capacity to produce up to 1.2 million kits/year, utilizing a mix of automated and manual processes. Phillips-Medisize combines components molded in-house with additional outsourced components to build a finished kit that is sealed and ready for delivery to the distribution center.
Collection Kits are stored at room temperature, away from heat and direct sunlight.While in controlled warehouse storage, the temperature range is maintained between 15°C to 30°C.
Unique logistics strategy
Mindy Bennett, Exact Sciences’ Director of Procurement, Planning and Distribution, says, “Working with UPS is an integral part of Exact Sciences’ business and everyone the company has worked with at UPS, from the drivers to management, has been the definition of resourceful, responsive and professional.”
Prior to FDA approval, UPS came to the table to help the company craft a unique logistics strategy tailored to transporting the test in a simple and efficient manner. UPS was willing to create a brand new model of logistics with Exact Sciences, while providing service to both the company and Cologuard patients.
Turek-Etienne explains, “Partnering with UPS has enabled Exact Sciences to follow through on its goal of getting doctors results within two weeks so that they may discuss them with patients in real-time. It’s a challenging task, and UPS took a leap of faith with the company to design a process for something that had never been done before, and that partnership has enabled Exact Sciences to deliver great service.”
In just 17 months on the market, Turek-Etienne notes, “Cologuard has achieved a patient compliance rate greater than 70 percent; this exceeds the most recent national screening compliance data and is double the rate for conventional screening methods, including colonoscopy, indicating that patients are more likely to follow through and get screened with Cologuard than other testing options.”