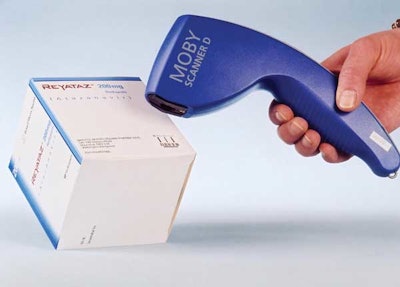
Bristol-Myers Squibb is among the first pharmaceutical companies to use DNA codes to fight counterfeit drug packaging. For more than a year, the company has been labeling anticancer drugs with DNA codes. According to Germany’s identif GmbH, a subsidiary of november AG, the codes make pharmaceutical packaging “forgery-proof.”
Each code is a unique sequence of synthetic DNA. For a label, like the one shown here, the DNA is mixed with a clear varnish laid down on the label substrate in one station of a flexo or offset press. The DNA can also be added directly to a folding carton, for example, by mixing it with the ink sprayed on by an ink-jet printer.























