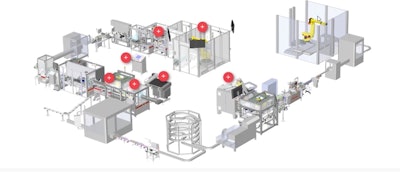
The system, which operates at a rate exceeding 150 parts/min, receives several components of a medical device, inspects them, assembles the components together, and processes the devices through several packaging operations. The first of these operations is flow wrapping, which includes insertion of a desiccant followed by hermetic sealing. Following a leak detection module that checks seal integrity and then a checkweighing operation, the sealed devices are moved into an MGS cartoner. Two literature inserts are added and all components are loaded into the cartoner. Cartons are sealed and move to a printing and inspection module where serialization is initiated. Overall the system integrates serialization and three levels of aggregation using a well-known serialization solution provider.
























