“Improper dosing is one of the biggest problems in giving acetaminophen to children,” says Sandra Kweder, M.D., deputy director of the Food and Drug Administration’s Office of New Drugs.
In a recent online article about the safe use of acetaminophen, the agency said an advisory panel in May recommended a reduction of strengths of liquid, chewable, and tablet forms to just one strength from seven; that dosing instructions to reduce fever be developed for children as young as six months; that dosing instructions be based on weight, not just age; and that standards be set for dosing devices.
“FDA is considering these recommendations,” says Kweder, in the article, “and for those that the agency adopts, we will work with manufacturers to try to get them in place on a voluntary basis.” FDA’s Web site notes, “The process of getting a regulation finalized could take several years, so having the drug industry act voluntarily would help make acetaminophen safer sooner.”
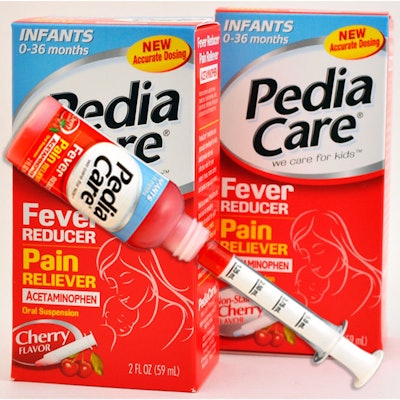
An example of a manufacturer doing just that is Prestige Brands, Inc. The marketer, seller, and distributor of over-the-counter healthcare and household cleaning products, with offices in Irvington, NY, and Jackson, WY, recently made some key packaging changes to its PediaCare and Little Remedies offerings to protect infants and children under the age of two.
“PediaCare and Little Remedies products have consistently been available for parents over the years,” says Albert Hwang, vice president, OTC products for Prestige Brands. “The voluntary changes to single-concentration acetaminophen dosing demonstrate the continued commitment to accuracy, safety, and innovation from PediaCare and Little Remedies.”
The company says these children’s pain relievers will also offer additional product enhancements, including age-appropriate dosing devices: Infant products will now contain a special dosing syringe and flow restrictors on the bottles; children’s products, for ages two to 11 years, will have the bottles with flow restrictors and will continue to contain dosing cups. Both infant’s and children’s formulations will continue to have weight-based instructions on the package.
“While the new single concentration for PediaCare and Little Fevers infants’ and children’s acetaminophen products will be among the first brands to hit store shelves, parents should know that there will be a ‘transition period’ during which the existing concentrated infant products and the new standard strength acetaminophen infant PediaCare and Little Fevers products may be on store shelves simultaneously,” says Hwang. “The new infant formula is less concentrated ,and the dose is therefore more than in the older infant formulation. And while reading and following package directions is always recommended to obtain accurate dosing instructions, it will be even more important while the two concentrations are available.” Parents and caregivers should ask a healthcare professional if they have any questions, he adds.
The new PediaCare and Little Remedies products were rolled out in late July at drug stores, supermarkets, and retailers nationwide.
Packaging specifics
Dean Siegal, Prestige Brands’ director of communications, said the package change was an industry-lead initiative, “guided by the Consumer Healthcare Products Association.”
Prior to changing to the new dosing feature, he says the infant products employed a syringe for concentrated drops (it now uses a press-in bottle adaptor). For children’s products, says Siegal, “a flow restrictor was added to the bottle to reduce accidental ingestion.”
The dosing syringe, he notes, is a two-piece device, with a polypropylene barrel and high-density polyethylene plunger. No supplier details were provided. Packaging is done by a contract packager.
Pricing has not changed with the new dosing feature, with costs ranging from the $5.49 to $8.99. Asked about consumer reaction and potential sales gains since adding the dosing device, Siegal says it’s too early to tell. “We’ll review it after the cough and cold season,” he adds.