The Healthcare Compliance Packaging Council (HCPC) awarded the winners of its annual Compliance Package of the Year competition at the organization’s 18th Annual National Symposium on Patient Compliance, held May 5 in Valley Forge, PA.
HCPC’s Compliance Package of the Year competition has been an annual event since 1995 and consists of two categories: one for trade packages released in the past year and the other for innovative designs not yet used commercially. The results for 2009 are:
• 2009 Compliance Package of the Year: Pfizer Toviaz™ Physician Sample
• Innovative Design: VitalityTM GlowCap™
• CPY First Runner Up: Bristol-Myers Squibb Onglyza™ Physician Sample
• CPY Second Runner Up: GlaxoSmithKline Lamictal® ODT Titration
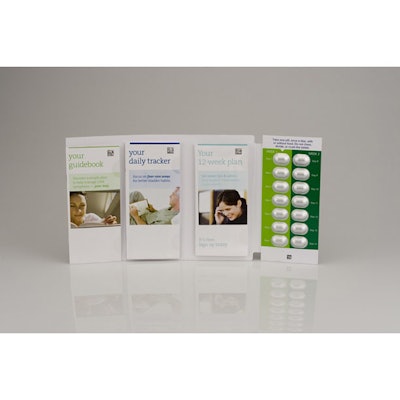
Toviaz Physician Sample
Launched in October of 2009, the Pfizer Toviaz Physician Sample is indicated for the treatment of overactive bladder (OAB). The package utilizes the AmerisourceBergen Packaging Group (www.amerisourcebergen.com) IntuiDoseTM child-resistant, compliance-prompting design. The design has passed CPSC Child Resistant/Senior Friendly unit-dose testing, garnering an F-1 rating and receiving favorable scores for Senior dispensability.
Done in partnership with Anderson Packaging, the four-panel Toviaz wallet design provides a comprehensive approach to initializing overactive bladder therapy, utilizing a compliance-prompting package design integrated with extensive tools for patient education, tips and tools for a detailed treatment plan, and tools to facilitate patient-doctor interaction for managing symptoms. The four-panel folder format incorporates a patient guidebook complete with explanations of symptoms, recommendations for managing OAB, how to properly take medication, and ways to enroll in interactive programs. The package includes a daily tracker, functioning as a guide and diary to track goals and health improvement. The program also provides a tool to review progress with the physician over the 12-week course of treatment. A customer response card is integrated in the pack, allowing patients to complete a questionnaire and enroll in an interactive treatment program to ensure success. Finally, the pack includes full patient prescribing information. Tablets are laid out in a simplified 14-day regimen, including instructions about how to properly ingest the medication.
The IntuiDose package features a novel approach to providing the highest levels of child resistance while at the same time making the product easily accessible to adults. The simplified push-through design focuses on providing a consumer-friendly solution to medication management. The use of a unit-dose compliance-prompting package facilitates medication adherence and effectiveness of treatment, guiding patients to fill their prescription and maintain compliance over the course of treatment.
When reviewing the Toviaz physician sample entry, one of the judges commented, “The diary-like book, "Your Daily Tracker" is a useful tool for encouraging both drug compliance and healthy habits. The carded blister is well laid out and easy to follow, and the foil doesn't break, which is often an annoyance. It’s compact and another two weeks could easily be added to this package for a monthly regimen format.”
Innovative Design: Vitality GlowCap
HCPC’s Innovative Design category was awarded to Vitality, Inc. for its GlowCap. The intelligent pill caps use light and sound, which can be followed by timely phone calls or text messages to remind people to adhere to any prescription regimen. Each time the pill bottle is opened, adherence data is securely relayed to Vitality over AT&T’s cellular network. This daily adherence data is used to automatically refill prescriptions as pills deplete. In addition, progress reports are sent to patients, caregivers and family members for increased social support. Using sophisticated pattern recognition, Vitality determines the unique motivators for each person and implements individualized behavioral programs to cost effectively lift adherence.
First Runner-Up: Bristol-Myers Squibb Onglyza Physician Sample
The First Runner-Up Compliance Package of the Year, BMS’ Onglyza Physician Sample, was also submitted by Anderson Packaging. Receiving FDA approval in July 2009, Onglyza™ is indicated for the treatment of type-2 diabetes. The two-panel Physician Sample folder design utilizes a cold-formed “foil-foil” unit-dose blister design sealed into a carded blister. Patient prescribing information is integrated into the package at the point of dispensing. A pocket is included in the design to facilitate compliance supporting literature, allowing for placement of wallet reminder card that directs patients to the drug Web site or toll-free number for further information about treatment and potential savings when filling their script.
Second Runner-Up: GlaxoSmithKline Lamictal ODT Titration
Receiving FDA approval in May 2009, the GlaxoSmithKline Lamictal® ODT™ Patient Titration Kit was made available commercially for the treatment of epilepsy as well as for bipolar disorder. The Lamictal package was also submitted by Anderson Packaging.
The compliance-prompting and child-resistant format of GSK’s Lamictal ODT medication utilizes a hybrid of the MWV Dosepak™ design, with a unique construction to allow for the needs of delicate tablets. The design has passed CPSC Child Resistant/Senior Friendly unit-dose testing, garnering an F-4 rating. The Lamictal® Patient Titration Kit is part of a family of Lamictal® products. Individual therapies are distinguished by package color schemes, including orange, green, and blue, with three titration therapies as well as four maintenance therapies. The orange therapy includes a titration of the following strengths; 25mg, 50mg, and 100mg tablets in a five-week regimen. Weeks of therapy are distinguished with concise graphics and patient dosing instruction, divided by individual peelable blister units contained within the package. Opening instructions with pictorial diagrams instruct patients on how to access tablets and ingest the product. Also prominently highlighted on the package are warnings about other drug interactions.
HCPC’s Compliance Package of the Year competition has been in existence since 1995. While there is no fee or other monetary requirements to participate, qualifying packages for HCPC’s Compliance Package of the Year “trade” category must: 1) be in a unit-dose format; 2) have at least one compliance-enhancing feature; 3) have been commercially available—anywhere in the world—at some point during [that year]; and 4) not require drug products to be “re-packaged” by patients. A category for Innovative Design recognizes compliance packages not yet commercially available.
The Compliance Package of the Year winner, the First Runner-up and the Innovative Design winner will be asked to designate scholarship funds to a university-level packaging school focused on the pharmaceutical industry. Complete competition guidelines are available at www.hcpconline.org, or by contacting the HCPC offices at 804/338-5778.
The Healthcare Compliance Packaging Council is a not-for-profit trade association whose mission is to promote the greater use of compliance-prompting packaging to improve patient adherence and patient outcomes.