It’s easy to learn a lot by looking at statistics for enforcement actions by the U.S. Food and Drug Administration. It’s harder to know what you learned.
Enforcement statistics, such as data on the numbers of inspections, Warning Letters, recalls, seizures, injunctions and criminal prosecutions, can appear to support multiple trends at once.
Also, they just tell part of the story. That’s because the FDA is really two agencies (at least) in one. When it takes enforcement actions, it’s a cop. But it’s also a licensing body, the gatekeeper deciding which new drugs, medical devices, food additives and other products are appropriate for entry into the marketplace. In this role as gatekeeper, the FDA also sets standards for products and activities, often with substantive input from the public, including the affected industry.
It’s always been a fascinating combination of roles for FDA, this cop and licensing body. And it’s easy to see that they are not altogether compatible roles: the cop is essentially adversarial towards the regulated industry, but the licensing body often works side-by-side with it, consulting and communicating to assure that the requisite information is generated.
When FDA dials up the toughness on enforcement against a given company, that’s going to make those new-product discussions a bit awkward. If, on the other hand, FDA-the-enforcer decides, as it does now and then, to emphasize informal pressure and voluntary industry action to obtain compliance, rather than tough enforcement measures as such, that tends to look more like the “partnership” model that usually prevails in the agency’s licensing and standard-setting functions.
So how do we judge FDA’s performance? If we’re judging FDA-as-enforcer, we look at their pattern of enforcement actions and count up inspections, Warning Letters, recalls, seizures, and criminal prosecutions. But if we’re judging FDA-as-licensing body, we examine how quickly they review New Drug Applications and other filings. We ask if those new product clearances appear to have been made hastily or mistakenly. We consider how effectively FDA had taken a leadership role in setting standards for new technologies or problems.
In this column, I’m considering the FDA in its enforcer role, and what we quickly see is that the picture of FDA’s recent enforcement patterns is mixed. Criminal arrests and convictions and voluntary recalls were up in 2005 over 2004, but Warning Letters were down. (All years referred to are fiscal.) Injunctions were up to 15 from 13 in 2004, and so were seizures, to 20 from 10 in 2004.
Congressman Henry Waxman of California thinks he knows what the numbers show. He issued a report called, “Prescription for Harm: The Decline in FDA Enforcement Activity.” Waxman has long been an active overseer of FDA from his Congressional seat, and these days, as a Democrat, he’s speaking from a minority position, so what he says gets considerably less media and Congressional attention than it did during years when Democrats had the majority in Congress. His office issued the June report based on its study of FDA enforcement in the George W. Bush years.
Waxman’s report alleges that its review of FDA records reveals that “FDA enforcement actions have declined under the Bush Administration,’” citing Warning Letters down 50% between 2000 and 2005, and seizures down 44% in the same period. He’s right on those numbers, since although there were more seizures in 2005 than 2004, there were fewer Warning Letters in 2005 than 2004, and both were well off their levels in 2000.
Among the other allegations leveled by the report is that FDA field staff has repeatedly recommended enforcement actions against companies whose products are in violation, only to be overridden by FDA headquarters. FDA has not yet issued an official response to the Waxman accusations, so it’s not clear how that allegation will play out.
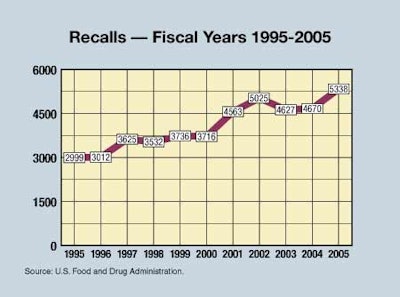
Waxman’s report noted that recalls were up, but wasn’t impressed. Even though recalls were up a substantial 44% since 2000, the report says, “Since one of the goals of an enforcement system is to deter violations and keep dangerous products off the market, the increase in recalls is not a hallmark of effective enforcement.”
On the other hand, maybe it’s a hallmark of increased voluntary compliance: recalls are voluntary but are often undertaken by a company under threat of serious enforcement action by FDA. Maybe recalls are up because of increased FDA pressure, or maybe due to enhanced company vigilance in the face of product problems. Remember too, that, say what you will about the odds of FDA coming after any particular company for a product violation, there is always the threat of substantial personal injury lawsuit liability to inspire companies to act cautiously when they might not otherwise be inclined to do so. Companies often have that firmly in mind as they decide whether to recall a product.
The report doesn’t think the reductions in enforcement activities can be attributed to “Increased compliance by manufacturers,” because the number of “inspectional observations” of violations that FDA inspectors record has not been going down in the past 5 years, says the report. Incidentally, FDA’s latest figures show that its number of inspections, 19꼃 in 2005, is down from 21꼅 in 2004, but is a little higher than 2001’s 18깩, and down from a peak of 22귿 in 2003.
What’s booming are “Import refusals,” up to 54긡 in 2005 from 48궉 in 2004. The numbers have been rising each year since at least 2001, when there were 18꼆 refusals. This likely reflects enhanced protection relating to anti-terror measures.
As we examine these figures, it’s well to remember that FDA continues to operate under an Acting Commissioner, who took over for a predecessor who now faces a whiff of scandal. Critics like Congressman Waxman might find the agency putting more energy into enforcement once its priorities get more clearly defined under the next permanent commissioner. In the meantime, the enforcement data are available for analysis. And debate.
Eric can be reached at [email protected]