From LPS Industries, LLC (www.lpsind.com), the pouch is made from a structure that includes, from the outside-in, polyester/solventless adhesive/aluminum foil/solventless adhesive/low-density polyethylene. Total thickness is about 4 mils. The outer polyester layer is printed flexographically in two colors. LPS preferred not to identify material suppliers.
LPS converts the materials into a three-side-sealed pouch that measures roughly 3”x8”. Pouches are shipped to Seattle-based PATH (Program for Appropriate Technology in Health), an international nonprofit organization based in Seattle whose mission is to improve the health of people around the globe.
Pharmaceutical manufacturer Boehringer Ingelheim donates its Viramune brand of Nevirapine (NVP) in opaque high-density polyethylene bottles that contain about 30 to 35 0.6-mL doses. The oral suspension requires no refrigeration, but must be given to the infant within 72 hours of birth, ideally 24, to help prevent the transmission of HIV, which typically occurs as the baby comes into contact with the mother’s blood during labor, according to Steve Brooke, PATH’s commercialization advisor.
“Nevirapine has been in use for years,” says Brooke. “It is the ‘gold standard’ used around the world. It is the simplest, least costly, and most effective drug for preventing the maternal-to-child transmission of HIV. So it is what the World Health Organization, UNICEF, and other organizations recommend.” Brooke explains that PATH became involved in the project through the United States Agency for International Development, the agency that funds the project that was initiated in the 2000-2001 timeframe.
Single, portable dose
The problem was that many mothers in Kenya deliver babies at home and don’t visit a clinic until the child’s first immunizations are due, several weeks after delivery. Clinics were having difficulties getting doses of NVP out to mothers. “I made a trip to Kenya and found there were nurses in some clinics who would fill the small amount of Nevirapine in an oral syringe—no needle—then wrap it in foil and give it to the woman to take home,” recalls Brooke. “Others would wrap it in tape or they would make use of whatever materials they had. Clinics were afraid of passing out this dose to give to the mothers to take home because they didn’t feel it was protected.
“We realized what would be helpful is just to make a simple standardized pouch that would help protect the dose when it’s at the mother’s home,” Brooke continues. “It also gave the opportunity to put some simple instructions on it.”
Brooke explains that providing a self-adhesive capability to the pouch was critical. “A lot of these clinics may not have reliable electricity for heat-sealing,” so manual sealing would be necessary.
Promising pilot program
In February 2006, PATH began a pilot program in 13 facilities spread out among five provinces in the country. The program, which lasted through June, was supported by USAID, with collaboration from the Elizabeth Glaser Pediatric AIDS Foundation and Family Health Intl.
When a nurse is counseling a pregnant woman who is HIV positive, she or he prepares a kit for the woman to have at home, to be ready if she gives birth at home. The kit consists of the two 200-mg tablets for the mother to take when she goes into labor. They are blister-packed by Boehringer Ingelheim.
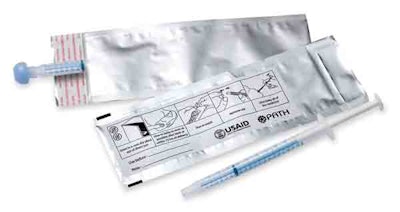
The nurse draws a 0.6-mL dose of Nevirapine syrup for the infant into an Exacta-Med dispenser manufactured by Baxa (www.baxa.com). A cap is placed on the dispenser tip and the nurse places it into the foil pouch.
“It uses a double-sided sticky tape with a peel-off protective sheet,” Brooke explains. “Then you fold over the end of the pouch and the adhesive seals it shut.”
Brooke believes, “This is a much better package for the patient to be able to take home. The package instructions include a series of drawings that show graphically to store the pouch in a cool, dry place. There’s an image of holding the baby and an image of how to take the drug out of the foil pouch and squirt it in the baby’s mouth.”
The pilot program, which involved 53 health workers and 543 doses, found that 93% of providers found it easy to use, and 98% of HIV-positive mothers interviewed reported liking the infant-dose pouch. Better yet, 100% of the moms interviewed said their infant received the NVP dose, either in a facility or at home.
Brooke reports that PATH is pleased with the pouch as well.
It’s now been adopted by the Kenyan government to be distributed through all the 3,000 clinics in the country that provide services to HIV-positive people.
Among the comments from healthcare providers using the infant-dose pouch in Kenya:
• “This pouch is a definite improvement to the current packaging of Nevirapine syrup, and my desire is to have all the PMTCT (prevention of mother-to-child transmission) sites under my coordination provided with the same.”
• “The pouch is only one, unlike the previous time when we used to use layers of wrappings—therefore it is easier to use.”
• “The Nevirapine pouch has made my work easier because the clients are impressed by the way it looks and are therefore keener to listen to me.”