With FDA food safety requirements and inspections on the rise in response to the Food Safety Modernization Act (FSMA) deployment, the first question for most people involved in the food industry is: Who does this law affect? In reality, it affects everybody from one end of the supply chain to the other. Primarily it will affect food producers and processors, as they will be tasked with identifying where the risks are in their systems and controlling them.
That’s where automation comes into the picture.
The following advice on how to leverage automation, for food manufacturers as well as food equipment manufacturers, summarizes remarks delivered to the industry by Dr. David Acheson, an expert on industry and regulatory matters who has contributed elsewhere in this Playbook.
Production tracking
The food industry has long struggled with product tracking, especially since the Bio-Terrorism Act enacted in 2005, which required product tracking one “step” up and one step back in the supply chain, a requirement that remains in the FSMA.
To protect a brand, food processors and packagers need to truly understand the safety and security of the supply chain. For example, if you are relying on imported shrimp from China, what do you know about the shrimp farmer? What do you know about the drugs that he is putting in that pond to control bugs and keep the shrimp healthy?
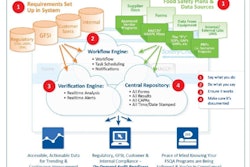
If you don’t know the answers to these questions, you are at risk. That’s why product tracking in supply chain systems is critical. The new law is going to require you to know more about risks in your supply chain, and you will likely have to be able to show through some form of documentation process exactly what you are doing to control those risks.
Keeping records is also important within your own four walls. As an example, if your operation involves roasting nuts, you have raw nuts going in one end of your roasting process and roasted nuts coming out the other end. What matters are the temperature of the roaster, the speed of the belt through the roaster, and the depth of the nuts on that belt. If the belt’s moving too fast, the nuts won’t get cooked enough. If the depth of the nuts on the belt is too deep, then the ones underneath won’t get enough heat.
With production tracking software, it’s simple to monitor, react, and record all this information on a continuous basis. You simply have to monitor these three factors to know when something is going out of spec so that you can take corrective actions, and you’ll have recorded verification that the corrective actions have worked.
Packaging and equipment
The bottom line is that food companies are looking to minimize risk—not just compliance risk, but safety and quality first and foremost. And that means that to satisfy all three issues— compliance, safety, and quality—the legacy equipment in place throughout much of the industry will need to be upgraded or replaced.
Four areas to focus on with equipment include:
-
Perform any equipment upgrades with validation in mind. The equipment will need to be able to validate that you exposed the product to enough heat to kill the agents of concern such as Salmonella and verify that it is working and capturing critical production/processing data elements.
-
Validation capabilities also need to address equipment cleaning. With allergens, for example, a food company will typically run products containing allergens at the end of a day or at the end of a run; but then you need an effective and documented cleanup process before you run a product through the system with no allergens.
-
Recognize that packaging equipment comes into contact with food. The notion that packaging is an inert item in your production process won’t fly any more. Machinery comes into contact with food. As such, this is a relevant risk that the FDA now recognizes and around which documentation needs to occur.
-
Labeling control (i.e., a product is not correctly labeled with regard to its contents) is another issue falling under tighter control with the FSMA. This is an especially critical matter on the subject of allergens. This is a simple issue to address with a product tracking system.
Liked this article? Download the entire Food Safety Playbook here.