As the world’s supply chain for pharmaceutical drugs comes under increasing scrutiny for safety and quality, Chromatic Technologies Inc. (CTI) has introduced printable thermochromic technology that is free of Bisphenol A, F and S.
The development meets stringent regulatory standards for pharma packaging.
The spread of the coronavirus has refocused the world’s attention on proper care of the supply chain for vaccines and drugs. Additionally, interest has surged in low-cost technology that monitors the cold chain, identifies temperature abuse and tampering and provides authentication.
Bisphenol A (BPA) has been labeled “an endocrine disruptor,” though it is found in many plastics, the lining of food and beverage cans and carbonless paper. Several companies have attempted to limit Bisphenol A, F and S in order to be more cautious.
CTI recognized that effort and developed these inks now available to pharmaceutical packaging companies.
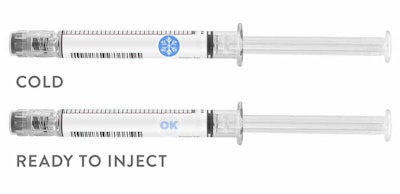
CTI’s BlindSpotz™ technology is a portfolio of patented technology that created low-cost printable sensors for drugs that detects freezing, thawing, gradual warming, tampering and authentication.
Pharmaceutical printers, as well as food and beverage printers, are requiring thermochromic inks to be free of Bisphenol A, F and S. Some printers have previously rejected traditional thermochromics for not meeting strict internal and external regulatory standards.
Beyond creating products free of BPA, F and S, CTI installed a 21-step checklist to validate quality and performance.

























