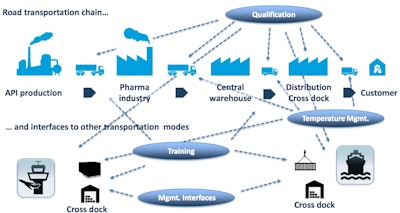
The road transportation supply chain is extremely complex, between APIs, manufacturing, central warehouses, distribution centers and customers. As Achim Bundschuh, Senior Advisor Pharma Logistics at QP Works, explained at PDA's 2016 Pharmaceutical Cold and Supply Chain Logistics Conference, there are many service providers and interfaces, which make logistics impossible to plan in the way that production steps are planned.
Bundschuh performed an analysis on over 100 GDP audits executed from 2013 to 2016 in Europe to provide a status report on challenges in GDP compliance and how companies can improve. Most of the companies—around 70%—were mid-size, family-owned companies with their own fleets.
The good news, Bundschuh said, is that the number of findings per audit is decreasing, with an approximate average of 25 in 2013 down to around 12 in 2016. However, there are still some major issues to resolve. He categorized audit findings into the following categories, listed in order of most findings to least:
-
Equipment/Vehicles: qualification, sensor calibration, temperature monitoring, sanitation/hygiene, maintenance
-
Supplier Assessment: audits, risk based transport assessment, equipment, validation, written procedures, measures and control
-
QM System/Document handling: QM-responsible for pharma, pharma-relevant SOPs, handling invalid docs, release process, self inspection
-
Deviation Management/CAPA: principle understanding, SOPs, written procedures, information of customer/contract provider
-
Training: document handling, deviations, hygiene, sanitation
-
Warehouse: qualification, temperature management, access control
-
Computer System: validation, written procedures, storage of data
In the first few years, some logistics service providers (LSPs) did not really have a grasp on qualification—the type of documentation and planning needed—and that it wasn’t just a slip of paper to file. In 2015 and 2016, he saw positive changes in quality and deviation management, with many LSPs maintaining a person dedicated to pharma logistics. “The mindset has changed a lot,” he said.
Bundschuh went over the following areas with room for improvement:
-
Though in-house training has improved, more can be done in terms of training at the interfaces between different players.
-
There has been an increased level of standardization in the utility vehicle industry, but there are still fleets without adequate qualification.
-
Dedicated pharma warehouses are usually qualified, but cross-docks are not always compliant. He noted that while warehouses may be compliant in terms of temperature or storage time, security must be looked into. In one instance, he was able to walk into a warehouse without being stopped or questioned.
-
While supplier agreements may be robust on paper, there may be a lack of control and measurement to ensure that reality matches the contract. He advised companies to visit contractors and subcontractors, and not just at the headquarters.
-
Validation of computer-based systems is becoming more of a focus, particularly in systems with direct impact to product quality. He recommended that people ask suppliers for computerized system validation documents.
-
There is always room for additional training. Ensure training is robust and kept up to date, and perform training jointly with partners in the supply chain wherever applicable.
Bundschuh noted that the fulfillment of GDP requirements might not be attributed to company size, but on the “will-level” and “skill-level” of the company as a whole. Small companies with high will-level improved, while bigger service providers were not always so willing to change their internal procedures. The role of each employee cannot be understated. “You need to reach the person at the wheel,” he said.

























