So far in RxTrace, I have explored the possible implications of the California pedigree law on drug repackagers, third-party logistics, and vendor-managed inventory (VMI) relationships and, of course, wholesale distributors. Now let’s take a closer look at the murky world of contract manufacturing organizations, or CMOs. I call this world “murky” only because you have to look very carefully at the relationship between a CMO and the contracting manufacturer to fully understand how the pedigree law might be applied. And even then you are going to have to check with the California Board of Pharmacy because the law is so confusing in this area.
First of all, I am not a lawyer. I am not qualified to tell you how to interpret the law and I have not confirmed any of this with the Board of Pharmacy. That’s your job. So be careful. Before you take any action that might affect your business, please check with your lawyer and/or the California Board of Pharmacy for official interpretations.
What the California law says about CMOs
California law defines contract manufacturers as “manufacturers.” It also includes 3PLs and repackagers in the definition of a “manufacturer” (see the California Business and Professions Code), article 2 “Definitions”, section 4033 “Manufacturer”). Here is that definition in its entirety:
“4033. Manufacturer (a) (1) ‘Manufacturer’ means and includes every person who prepares, derives, produces, compounds, or repackages any drug or device except a pharmacy that manufactures on the immediate premises where the drug or device is sold to the ultimate consumer.
“(2) Notwithstanding paragraph (1), ‘manufacturer’ shall not mean a pharmacy compounding a drug for parenteral therapy, pursuant to a prescription, for delivery to another pharmacy for the purpose of delivering or administering the drug to the patient or patients named in the prescription, provided that neither the components for the drug nor the drug are compounded, fabricated, packaged, or otherwise prepared prior to receipt of the prescription.
“(3) Notwithstanding paragraph (1), ‘manufacturer’ shall not mean a pharmacy that, at a patient’s request, repackages a drug previously dispensed to the patient, or to the patient’s agent, pursuant to a prescription.
“(b) Notwithstanding subdivision (a), as used in Sections 4034, 4163, 4163.1, 4163.2, 4163.3, 4163.4, and 4163.5, ‘manufacturer’ means a person who prepares, derives, manufactures, produces, or repackages a dangerous drug, as defined in Section 4022, device, or cosmetic. Manufacturer also means the holder or holders of a New Drug Application (NDA), an Abbreviated New Drug Application (ANDA), or a Biologics License Application (BLA), provided that such application has been approved; a manufacturer’s third party logistics provider; a private label distributor (including colicensed partners) for whom the private label distributor’s prescription drugs are originally manufactured and labeled for the distributor and have not been repackaged; or the distributor agent for the manufacturer, contract manufacturer, or private label distributor, whether the establishment is a member of the manufacturer’s affiliated group (regardless of whether the member takes title to the drug) or is a contract distributor site.”
By the way, the references in this definition to sections 4034 and the various subsections under 4163 are all pedigree-related sections, so subsection (b) of this definition appears to be aimed squarely at defining who is a “manufacturer” with respect to pedigrees. And that covers a lot of entities in the U.S. pharma supply chain.
The law uses the term “contract manufacturer” within this definition of “manufacturer” but does not define it. Wikipedia’s definition of a “contract manufacturer”: “A contract manufacturer (‘CM’) is a manufacturer that contracts with a firm for components or products. It is a form of outsourcing.”
Whenever a CMO is involved, are they exempt from passing pedigrees?
In the U.S. pharmaceutical supply chain the use of CMOs as part of the manufacturing process is widespread, deep-rooted, and mature, so it is important to figure out how these relationships might impact the generation and passing of electronic pedigrees. A contract manufacturer is a manufacturer, and it is common knowledge that pedigrees in California must start with the manufacturer (after Jan. 1, 2015 and 2016), but we also know that pedigrees in California are intended to show only changes in ownership.
So in the simple case, if a “manufacturer” issues a contract to a CMO—also a “manufacturer”—to produce their drug, and the CMO subsequently ships the drug to the “manufacturer” in fulfillment of that contract, which “manufacturer” needs to start the pedigree? Look at the ownership. In this case, the CMO does not appear to own the drugs they are making since they are simply fulfilling an outsourced manufacturing function. In this case it appears that the ownership starts with the “manufacturer” that issued the original contract to the CMO. That’s what it appears like to me in this simple example, but check with your lawyer and/or the Board of Pharmacy to confirm.
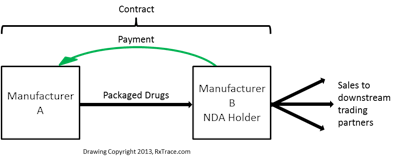
Figure 1 is a drawing of this simple case. Manufacturer B is the FDA New Drug Application (NDA) holder (or an Abbreviated New Drug Application (ANDA) holder for generic drugs) and they issue a contract to manufacturer A to produce finished packaged drugs to their specification on their behalf. B makes a payment to A for contract services rendered under the contract, but A never owns the drugs, so, according to that logic, B should probably start the pedigrees when they ship to downstream trading partners. The shipment from A to B would apparently not need to be reflected on those pedigrees because there was no change of ownership, but even in this simple, common business arrangement, I suggest that you confirm this interpretation with your lawyer and/or the Board of Pharmacy.
Figure 2 shows a subtly different situation. In this case, manufacturer C is the NDA holder. They have an exclusive contract with manufacturer D, a well-known NDA holder and maker of other drugs, to supply them with finished packaged drugs with D’s name on the label. Just like in the first case above, D makes a payment to C, but this time, because C is the NDA holder, D is apparently buying the packaged drugs. In this case, manufacturer D is apparently filling the role of an exclusive distributor of C’s drugs with the label indicating that the drugs are “distributed by” D.
Who starts the pedigree in this instance? It appears to me that C would need to start the pedigrees and D would need to be licensed by the California Board of Pharmacy as a wholesale distributor if any of their downstream customers are within the State of California, but check with the proper authorities to confirm. If true, then D would need to update the pedigrees that were started by C to reflect their receipt and their sale, just like any other wholesale distributor.
Figure 3 shows another similar, but subtly different situation. In this case, manufacturer F is the NDA holder. They have a contract with manufacturer E to make their drugs, package them in bulk form and ship it to them. F then packages the drugs into finished goods form and sells them to downstream trading partners.
In this case, my best guess is that F, being the NDA holder, is simply using E as a CMO, so just like the first case above, the bulk packages of drugs shipped from E to F would not need to be serialized or pedigreed since no ownership change has occurred and F is going to package the drugs for distribution into the supply chain. The pedigrees would be first created by F only when they sell to downstream trading partners. Again, check with the proper authorities to confirm.
Is this starting to look easy? Ok, now look at Figure 4, which shows a subtle variation on the previous case. The only difference in this case is that manufacturer G is now the NDA holder. Manufacturer H issues a purchase contract with manufacturer G to supply them with bulk pharmaceuticals. H then packages the drugs into finished good form and sells them to downstream trading partners.
In this case, it appears to me that, because H is buying bulk drugs from the NDA holder, this now represents a change of ownership. Does the fact that the drugs are in bulk form make any difference? I don’t think so. In this case, G would probably need to serialize and pedigree their bulk containers and H, who is acting as, surprise, a repackager, would need to serialize their finished goods packages and update the incoming pedigrees to reflect the linkage to the serial numbers of the incoming bulk packages for their outgoing pedigrees (see “Repackaging Drugs Under A Serialization Regulation”). California licenses repackagers as wholesale distributors. This is my uneducated interpretation, please get an official interpretation from your lawyer and/or the Board of Pharmacy.
But these are all “manufacturers.” Why are some treated as wholesalers?
Good point, but California does not only require manufacturers to create ePedigrees, they must also update them just like any other downstream trading partner if they buy the drug from an upstream trading partner. Here a partial extract from the California definition of “Pedigree.”
“4034. Pedigree (a) ‘Pedigree’ means a record, in electronic form, containing information regarding each transaction resulting in a change of ownership of a given dangerous drug, from sale by a manufacturer, through acquisition and sale by one or more wholesalers, manufacturers, repackagers, or pharmacies, until final sale to a pharmacy or other person furnishing, administering, or dispensing the dangerous drug. …”
I have italicized the key language above that implies that a pedigree must include information regarding transactions resulting in a change of ownership where a manufacturer acquires a drug through a purchase. See if your lawyer agrees with this interpretation.
I purposely called all of the entities in my drawings above “manufacturers.” The reason is that so many companies that most of us recognize as drug manufacturers actually fulfill all of these roles. More times that you would expect, a single company can fill more than one of these roles—from A to H—for different products, all at the same time. You know these companies as “a drug manufacturer” and I know them as “a drug manufacturer,” and they think of themselves as “a drug manufacturer,” but for different products, they may actually be filling the role of an exclusive distributor, a CMO, or a repackager. Each of these would be treated differently under the California law with respect to pedigree origination and updating.
Figure 5 shows a hypothetical “manufacturer X” that serves every one of these roles for different products. Here is the breakdown:
• A to X. For these products, X is the NDA holder so A appears to be serving as a CMO/CPO under contract with X.
• X to B. For these products, B is the NDA holder so X appears to be serving as a CMO/CPO under contract with B.
• C to X. For these products, C is the NDA holder so X appears to be serving as a wholesale distributor, perhaps an exclusive distributor or a 3PL.
• X to D. For these products, X is the NDA holder so D appears to be serving as a wholesale distributor, perhaps an exclusive distributor or a 3PL.
• E to X. For these products, X is the NDA holder so E appears to be serving as a CMO under contract with X.
• X to F. For these products, F is the NDA holder so X appears to be serving as a CMO under contract with F.
• G to X. For these products, G is the NDA holder so X appears to be serving as a repackager.
• X to H. For these products, X is the NDA holder so H appears to be serving as a repackager.