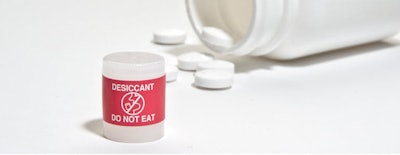
Clariant, through its Healthcare Packaging business unit, introduces desiccant canisters with bright red labeling to better differentiate them from drug products.
Displaying an easy-to-understand pictogram and the words “Do Not Eat” in multiple languages, the colored labels offer a further option to support packaging safety.
Drop-in style desiccants, such as canisters and packets, are the most widely used method of controlling moisture in nutraceutical and pharmaceutical bottle packaging applications. According to the company, all desiccant canisters are marked with warnings to help avoid accidental ingestion, however, depending on the color of the drug there may not be a sharp contrast. The distinctive and vibrant color of the red canister label can help patients more easily recognize the desiccant.
The new labels are made using FDA- and EU-compliant materials and include an outer varnish coating, so ink is not in direct contact with the drug. The canister labels can also be customized for specific color-differentiation or with a customer’s specific branding.
Designed for high-speed insertion, Clariant's full line of rigid canisters has become the solution of choice when controlled atmosphere packaging is required to control moisture, oxygen, odor and other volatiles. The active packaging platform is customizable with various sorbent materials including silica gel and molecular sieve.
Clariant has the capacity to produce over 1 billion desiccant canisters per year at its plant in Belen, NM, USA. Construction has begun on a new plant in Cuddalore, India, which will also produce labeled desiccant canisters, supporting customers’ Business Continuity Planning (BCP).
”The safety of the patients using our customers’ products is paramount to our mission,” said Philippe Depois, Head of Sales, Clariant Healthcare Packaging. “By color-differentiating the canisters, we are adding another layer of safety to protect our customers and consumers.”


























