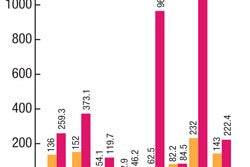
DMFs are confidential, proprietary assets that present to the FDA the formulae, processes, test methodology, and other data relevant to the manufacture of products used in the composition, packaging and processing of pharmaceuticals or biologics. Indian firms accounted for 104 out of the 251 new DMFs made in the US in April-June 2006, compared with 74 out of 213 a year ago.
Indian drug firms file most DMFs in U.S.
Indian pharma companies filed the largest number of drug master filings (DMFs) with the FDA in the April-June 2006 quarter, accounting for almost 42 percent of filings.
May 31, 2007
Machinery Basics
Annual Outlook Report: Workforce
Hiring remains a major challenge in packaging, with 78% struggling to fill unskilled roles and 84% lacking experienced workers. As automation grows, companies must rethink hiring and training. Download the full report for key insights.
Download Now
Annual Outlook Report: Sustainability
The road ahead for CPGs in 2025 and beyond—Packaging World editors review key findings from a survey of 88 brand owners, CPG, and FMCG readers.
Download Now
Downloads