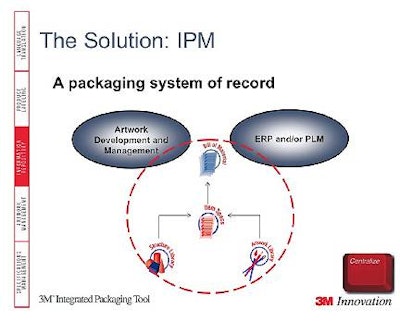
Approximately 50% of all recalls are due to packaging errors. Consumer safety cannot be compromised by mislabeled products, including usage instructions, allergen warnings, and ingredient statements. Life science companies cannot afford to compromise quality, in packaging or anything else. Information Technology systems that are document-based do not address critical packaging issues in life sciences. Integrated packaging management provides needed functionality to ensure top quality.Haldane said, “Many pharmaceutical companies have over-the-counter products, too.” He explained that consumer packaged goods companies have distinct packaging needs that if addressed can produce significant results, including:
Increasing speed to market Reducing packaging costs Enforcing brand standards. Complying with retailer mandates.Click here for a direct PDF format download of Haldane’s presentation. Click here for a list of all available presentations.