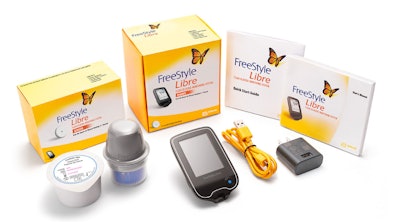
A recent FDA News Release article contained good news for adults with diabetes: finger pricks to obtain blood samples may soon be a thing of the past. The FDA just approved Abbott’s FreeStyle Libre Flash Glucose Monitoring System, a device that uses a small sensor implanted below the surface of the skin in lieu of fingerstick testing. The device continuously monitors glucose levels, which are determined by waving a mobile reader above the sensor.
The sensor is discretely worn on the back of the upper arm for up to 14 days and produces readings in just a second, even though clothing. Each scan gives the wearer a current glucose reading, a view of the last 8 hours, and an indication of which way they’re heading. The only real limitation is that readings have to be initiated by the wearer, so the device is unable to provide real-time alerts while the user is sleeping or not tending to the FreeStyle.