The International Safe Transit Assn. (ISTA) Standard 20 Revision 2.0 is a process standard developed by pharmaceutical industry experts. It is a design and qualification process that applies the quality-by-design (QbD) approach. The structure and path to design, test, verify and independently certify a specific Insulated Shipping Container (ISC) for use is provided. It sets the minimum requirements for qualifying insulated shippers and has been proven to develop shippers that meet regulatory expectations.
Standard 20 contains full documentation, examples of protocol, qualification and data reports, full instructions and testing and reporting criteria. With Standard 20, ISTA reached a new level by not only setting the standard, but by providing a highly structured context in which to perform testing.
What is the value of implementing Standard 20 to ISC suppliers and their customers?
When ISTA certification is in practice, pharmaceutical companies can then shop for ISC's based on simple and reliable criteria. They can have confidence that performance is not subject to uncertainty. ISTA certification opens markets for shipping containers to a wider range of suppliers. Cold chain strategies can be constructed based on known reference numbers for package performance.
• The standard allows for different ISC’s to be compared in an “apples-to-apples” way (when used in conjunction with ISTA 7E).
• Potentially reduced client development cycle due to client acceptance of existing standard 20 qualification data on off-the-shelf ISC’s.
• Ensures repeatability and accuracy.
• ISTA has taken a collaborative approach to developing Standard 20 and we believe have set the industry minimum requirements while recognizing that there can be more than one way to meet the standard.
• For someone without a qualification program or procedures ISTA has developed a process to get started.
• It provides a significant reduction in time, resources and money required to design, qualify and validate an ISC’s performance, providing a gain to any individual customer choosing an ISTA-validated and certified ISC.
• Standard 20 is the first and only thermal performance protocol developed by a broad range of industry experts from various disciplines that meets regulatory expectations for maintaining drug quality during shipping.
• Standard 20 provides a sound, scientifically justified process for determining comparative performance characteristics of ISCs.
End-user benefits
• Off the shelf, independently certified packaging solution that meets industry requirements with the requisite qualification data to support high-value pharmaceutical/biopharmaceutical manufacturers and global regulators.
• Supply chain optimization through selection of the most competitive ISC solution(s) for the most economical shipping lanes.
• Reduction in cost and time to market by selecting pre-qualified ISC designs. Internal resource demands are minimized and focused on product development activities.
• Internal laboratories benefit from improved efficiency in executing thermal performance testing.
Supplier benefits
• Supply markets are opened up and provide a more level playing field by providing designs that can be compared based on performance data, cost and service levels.
• Facilitates and encourages new product development and innovation as there is an industry-accepted methodology to demonstrate benchmark thermal performance for new ISC products.
Contract test lab benefits
• Industry standardization via ISTA 7E and Standard 20 deployment increases the demand for independent test laboratories certification activities (Standard 14); and the resultant value of the independent test laboratory within the marketplace is increased.
• Standardization and supporting qualification documentation improves the laboratory’s efficiency in completing high-quality work for their clients.
Behind the standard’s creation
One of the motivations behind creating this standard was to provide ISCs global comparability from design to design and manufacturer to manufacturer. Until now it has been difficult to know if the performance of one ISC is better or worse than another for a particular cold chain challenge. Adoption of Standard 20 and ISTA 7E (a real world set of shipping lane temperature data, which has been statistically analyzed to create a robust thermal profile) as global standards addresses this issue by enabling head-to-head comparisons.
So why did ISTA get involved in developing a process for the design and qualification of ISC’s?
ISTA has been around in one form or another since 1948, establishing performance levels for testing protocols that validate that a package design provides the proper amount of protection to products against the hazards they would be exposed to during shipment to the ultimate user.
With minor exception, temperature and humidity were only used as preconditioning before the actual testing was conducted. We knew these conditions influenced the performance of the packaging, but we did not consider them a hazard to the products. This started to change when the pharmaceutical industry realized that insulated shipping containers were needed and “cold chain packaging” emerged.
Pharmaceutical shippers and their packaging suppliers began to realize that temperature was a hazard that needed to be a test element all by itself. As a result, temperature performance profiles begin to appear. Questions ensued about where the profiles came from, how they were determined and were they accurate. Some pharmaceutical shippers and their packaging suppliers made an effort to establish a process to follow for developing temperature profiles and ISTA became involved in the effort. About this same time good manufacturing practices started to turn toward good distribution practices as well. So what test profile you used and what developmental process you used to engineer an insulated shipping container gained importance. Here again, the key industry parties realized the following:
• It’s all about data and processes
• It’s all about science-based processes
• It’s all about processes that are industry standards
Standard revisions
In 2008 ISTA partnered with the Parenteral Drug Assn. (PDA)’s Pharmaceutical Cold Chain Interest Group (PCCIG) to develop baseline standards for hot and cold profiles with the creation of a Temperature Profile Development Protocol (ISTA-001-08), which resulted in the ISTA 7E profiles.
ISTA then partnered with several pharmaceutical industry leaders to bring a companion standard to the performance of ISC testing titled Standard 20. Standard 20 sets forth, in comprehensive documentation, exact procedures for utilizing the 7E profiles in a test environment where the lab, the personnel and the protocol are certified and consistent. In 2014 Revision 2.0 was published. The following is an overview of what changed and what stayed the same.
What hasn’t changed in Standard 20 Rev. 2.0? The original guiding principle of Standard 20 has not changed. The premise is still the same—to have an industry standard so that users have a qualified insulated shipper that meets regulatory expectations. Standard 2D 20 is a process that is focused on regulatory compliance for the end user.
What has changed in Standard 20 Rev. 2.0? It was simplified, clarified and streamlined. The size and complexity of the original Standard 20 was reduced. The simplifications benefit both suppliers and users with an easy-to-implement approach to documenting shipper qualification.
In addition, the ISTA Thermal Council wanted to ensure all requirements were stated clearly, that all requirements provided maximum flexibility, and to tighten the thermal chamber performance requirements. The results simplified and streamlined the entire program by reducing the size and number of documents in the entire Standard 20 Rev. 2.0 program. In addition, protocol templates moved from an outline format to a table format.
Rev. 2.0 now clarifies thermal sensor locations. A table was created to precisely specify how many and where to locate thermal sensors in an insulated shipping container based upon the ISC design and payload configuration.
Other changes from user feedback included the following:
• More flexibility around use of other thermal profiles.
• Removed qualified air temperature data analysis (QAT).
• Made parts of the process optional.
• Required a physical test as part of DQ.
• Allowed physical qualification at any ISTA lab certified to execute ISTA 2B or 3A.
• Provided clear direction on what to do if a test fails.
• Removed specific company references.
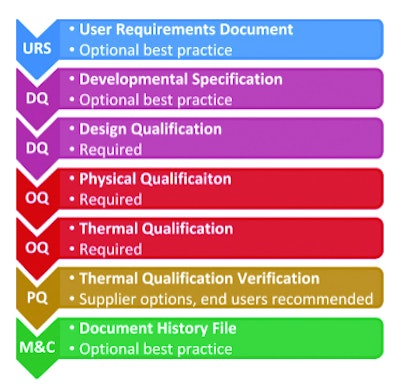
• Incorporated process flow diagram
Future challenges
So, what are the challenges ISTA faces in moving Standard 20 forward? The major challenge is gaining marketplace acceptance by establishing enough critical mass of suppliers providing Standard 20-developed ISC’s with commonly structured data packages to get the attention of the FDA and acceptance in audit reviews.
Realization by the pharmaceutical industry that their usage of Standard 20 as a pharma packaging design standard, with its resultant data packages, will ultimately yield FDA recognition of the process and everyone’s life will be better.
ISTA needs to show pharma packaging professionals the simplicity of implementation of Standard 20 into their co the smpany’s company’s Good Distributions Practices (GDPs).
Creation of an ISTA Standard 20 User’s Group with the mission to promote the value and benefits of implementing the process into a company’s Good Distribution Practices.
Editor’s note: Edward A. Church is ISTA President Emeritus and Eric Hiser is ISTA Vice President—Technical

























