Pharmaceutical manufacturers and their packaging suppliers deserve and receive recognition in the Healthcare Compliance Packaging Council’s (HCPC) annual Compliance Package of the Year competition, but the real winners are the patients who take these medications as instructed by their physicians.
Since the HCPC competition began in 1995, progress in compliance packaging has risen steadily, helping to meet the organization’s mission to improve patient adherence and outcomes through packaging.
This year’s winners clearly show that the packaging community and manufacturers “get it,” when it comes to understanding the patient’s perspective. That recognition is increasingly important given the large population of aging baby boomers who suffer from diseases such as diabetes and cystic fibrosis, which are addressed in the 2015 award-winning packages.
HCPC’s Compliance Package of the Year competition recognizes the most innovative pharmaceutical packages designed to improve patient adherence for the preceding market year. This year’s winners are:
• 2015 HCPC Compliance Package of the Year: Vertex ORKAMBI® Medication Package from PCI Pharma Services
• 2015 HCPC Compliance Package of the Year First Runner Up: Merck Januvia® Medication Package from Westrock
• 2015 HCPC Compliance Package of the Year Second Runner Up: Mannkind Afrezza® Medication Package from PCI Pharma Services
HCPC 2015 Compliance Package of the Year
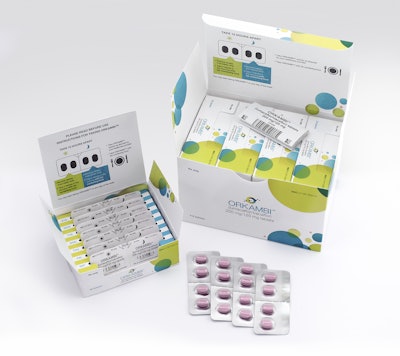
With its medication package from PCI Pharma Services, Vertex Pharmaceuticals’ ORKAMBI® (lumacaftor/ivacaftor) is a prescription medicine used for the treatment of cystic fibrosis (CF) in patients age 12 years and older who have two copies of theF508delmutation (F508del/F508del) in theirCFTRgene. ORKAMBI® packaging offers several compliance-prompting features for patients.
ORKAMBI® is provided in convenient daily blister units, with graphics clearly highlighting the AM and PM dosing requirements. Individual blister units are file-packed into a weekly organizer carton, with individual days neatly arranged to illustrate the daily regimen. Dosing instructions are positioned to educate the patient, reminding patients to take the AM and PM doses 12 hours apart and to take their medicine with fat-containing foods. Four weekly packs are provided in an outer monthly carton, which contains graphics that further reinforce dosing instructions as well as the FDA-approved labeling.
One of the judges reviewing the entries said, “Vertex Pharmaceuticals’ Orkambi pack for treatment of cystic fibrosis is quite simply one of the best pharmaceutical package designs I’ve ever seen. It starts with a folding carton that displays like a carryout lunch box. The carton opens from the top whereby the inner flap provides easy-to-read dosage instructions. Inside the box are four separate and identical folding cartons that all open just like the larger outer carton.
“All packaging components are color coded, with each of the four cartons representing a seven day supply of medications, four tablets per day. There is no wasted space. The true beauty of this package, however, is once you open each of the four smaller cartons. Inside, a color-coded carrier is marked with each day of the week. The carrier is slotted so that seven blister packs are cushioned within each slot, from both ends. The patient can look at the inside top flap on each of the cartons to see how to remove two tablets from the blister pack in the morning, and two more at night.”
HCPC 2015 Compliance Package of the Year First Runner Up
Westrock’s packaging is for Merck Januvia® is a once-daily prescription diabetes pill that, along with diet and exercise, helps lower blood sugar in adults with type 2 diabetes. The Januvia® Medication Package promotes patient medication adherence through the use of a custom design that integrates:
• A calendared blister, clearly labeled by day on each individual pill cavity, highlighting the four-week dosing regimen. Visual cues prompt medication taking and tracking.
• A 5/6th panel provides space for medication information and dosing instructions
• Branding for Merck / Januvia on the outer package’s readable, flat space
“WestRock and Merck partnered to design the Januvia package to help diabetes patients adhere to their medication,” said John Grinnell, VP, Sales, Health, Beauty, & Adherence, WestRock. “Packaging is the one tool that stays with the patient throughout the medication journey, making it uniquely effective in providing regimen support.”
The Januvia package builds on Westrock’s legacy of designing medication packaging that’s proven to enhance adherence, with adherence packaging shown to achieve an 80% Proportion of Days Covered with up to 37% more patients versus standard pharmacy vials.
“With this Januvia® 100 mg package, Merck & Co. demonstrates that it understands how to make medication adherence simpler for patients with Type 2 diabetes,” one of the judges remarked. “The package is easy to use, convenient and loaded with helpful features.”
HCPC 2015 Compliance Package of the Year Second Runner Up
PCI Pharma Services’ medication package is for MannKind Afrezza®, a rapid-acting inhaled insulin breathed through your lungs and is used to control high blood sugar in adults with type 1 and type 2 diabetes. Taken at the start of a meal using a specially designed inhaler, Afrezza dissolves rapidly upon inhalation to the deep lung and delivers insulin quickly to the bloodstream. Peak insulin levels are achieved within 12 to 15 minutes of use and help to control post-meal blood sugar spikes that affect HbA1C levels.
Afrezza® is provided to patients in color-coded prefilled cartridges. Individual cartridges are blister-packed in quantities of 15 based on a color-coding system to indicate the strength. Four unit-strength cartridges are blue, eight unit-strength cartridges are green, and 12 unit-strength cartridges are yellow. An indication of each cartridge strength is also molded in the plastic of the individual cartridge.
Two 15-count cartridge blisters are provided in an overwrapped pouch. The pouched unit reinforces the color-coding methodology by utilizing the drug strength colors in the package graphics, in addition to providing detailed instructions for refrigeration of the product as well as equilibration requirements prior to dosing, bringing the product up to room temperature. Pouch graphics identify that once a blister strip is opened to access the cartridges, they must be used within 72 hours.
Also provided to the patient are two prewrapped inhalers in a prepackaged carton, protecting the inhaler from dust, particulate, etc. Carton artwork provides detailed dosing instructions, a color-coded dosing chart based on the number of units needed per dose, as well as a calendar to guide the patient in timely replacement of the disposable inhaler unit. The carton also reinforces the storage requirements for Afrezza®.
The individual pouches and inhaler carton are combined with regulatory literature in the dispensing carton. Package graphics highlight the AFREZZA website, where patients can find additional resources including videos, storage and dosing instructions, prescription savings information, and social support for patients, as well as other resources.
Several of the judges noted that inhalation therapy can be a complicated process for patients but approved of the well-developed Afrezza package. “Providing a needle-free method of administering insulin has been a long-sought goal. But, beyond successfully introducing a method of accomplishing this, the Afreeza package successfully provides clear instructions and an inhalation device that provides a high degree of user friendliness: the color coding for different dosages; and the simplicity of the inhalation device. Care was taken with the patient guide to provide clear, understandable text and graphics for what must be a carefully administered therapy.”
Student competition winner
This year the HCPC launched a corresponding student competition to recognize innovative pharmaceutical packaging designs from undergraduate or graduate students of pharmaceutical and/or packaging schools that assists patients in taking their medication as prescribed. This year’s student winner is Kathryn Binsfeld, currently a sophomore at Michigan State University. Her Night and Day dual-chamber container allows patients to push down on a two-part cap that indicates AM or PM.
The patient pushes down on the appropriate cap portion, which releases a tablet/capsule into the bottom cavity. Once there, the user can then line the arrow up with the tick mark in order to gain access to the cavity, thus maintaining child-resistance. The cap resets itself once both AM and PM buttons have been pushed.
“Ms. Binsfeld submitted a very well thought out package and we are pleased to award her a $1,000 scholarship for the innovative design. It is exciting to see our future packaging professionals create packaging that will improve adherence and healthcare outcomes,” said Walt Berghahn, HCPC Executive Director.