Pharmaceutical makers face multiple packaging challenges, but topping their list of concerns are issues pertaining to readable and scannable bar codes, package security, counterfeiting, and Food and Drug Administration compliance and validation.
That’s according to pharmaceutical professionals responding to a mid-April survey on Packworld.com, Packaging World’s Web site. The survey asked packagers to identify what they see as the key issues regarding pharmaceutical and medical packaging.
“Too much information regarding product use, side effects, etcetera, is required to be shipped with products,” was a concern expressed by a production executive with a large midwestern firm. “The current printed product literature is expensive to ship out, and from what we see, most [consumers] discard this information upon unpacking the products. I would like to see this information stored and updated on a Web site so that customers could reference the site when product-related information is required.”
An engineering representative with a large organization in the mid-atlantic states noted that “implementing bar codes on all packages,” was his primary concern. Specifically, he referred to “engineering issues such as the quality of online printing, labeling, real estate, and scan verification.” A similar thought was cited by a quality assurance professional with a mid-sized midwestern firm. He was concerned with “bar-code scanning of individual units of packaging materials and the [FDA] Part 11 implications for the software and hardware used to perform these tasks.”
In the Northeast, an engineer with a mid-sized pharmaceutical company lamented, “Federal labeling regulations are causing increased packaging costs, and are probably not a necessity.” On a related note, a purchasing agent with a large midwestern business commented that “suppliers [of pharmaceuticals] are just about required to have vision systems to be able to detect missing copy and broken text. This is a large expense and limits suppliers that we will consider.”
Machinery issues
Bar coding of labels was noted as an important topic according to an engineer at another large company in the Midwest. “The FDA-issued requirement that all National Drug Code numbers for each product must be on labels [means] we’re currently evaluating the different bar-code types and the impact at all of our plants.”
Besides bar coding and labeling, one survey respondent felt a need to “develop a way to accurately count tablets or capsules of controlled substances at high speeds.” This engineer of a mid-sized firm in the Northeast added, “I expect the industry to continue to develop better optical counters.”
Another cautionary mention related to packaging machinery was voiced by a packaging representative of a large pharmaceutical company in the Northeast. He said, “One of our top concerns is that we are purchasing increasingly faster and more automated equipment that requires materials to be more uniform than they actually are. This causes a debate when there is downtime [as to] what is at fault—poor-quality packaging materials or equipment that cannot run with real-world quality. Both equipment and material suppliers need to improve and meet in the middle on this.”
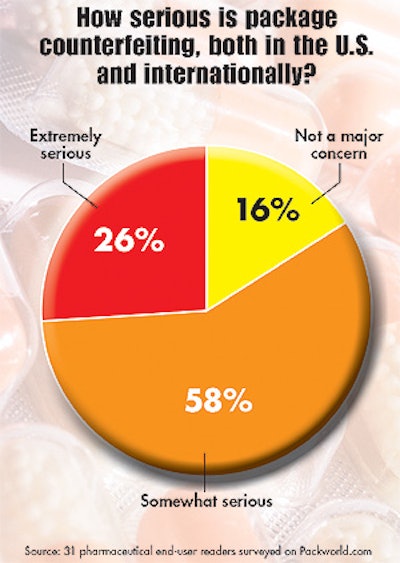
Security, counterfeiting
Respondents also noted that security and counterfeiting represented issues of importance. A quality assurance manager with a large midwestern pharmaceutical business noted that his firm “will complete the move away from neck bands to induction seals this year, even for over-the-counter” products. He said bottles with pinholes caused line downtime, suggesting a “possible quality program with suppliers or incentive-penalty program,” could alleviate the difficulty.
A purchasing representative with a smaller firm in the Midwest stated that “better packaging will be developed” to help maintain “security of the package content until it reaches the customer.” Seconding those beliefs was an R&D professional with a mid-sized midwestern company, whose primary issue was with “the safety seal not being secure.” His solution was “more reliable equipment and detection equipment.”
Up until now, counterfeiting has reportedly been more prevalent outside of the United States. But that didn’t keep survey respondents, most of whom are located in the United States, from being concerned about it. “Package counterfeiting [involves] using imitation or inferior product contents,” said the plant manager of a small firm in the West. He expressed faith that “reputable firms should and will develop antitheft inserts to provide validity regarding the package and contents.” And the quality assurance executive with a smaller Northeast operation believed “the industry will come up with a way to stop package counterfeiting.”
But a packaging representative with a mid-sized pharmaceutical concern in the Midwest didn’t share their faith. “I’m not sure what can be done,” he said. “It seems as though when we come up with an idea to deter counterfeiting, the counterfeiters have a fix the next day.”
Other hot-button issues mentioned by survey respondents were “verifying the integrity of product being packaged, and the ability to control purity, particularly of parental drugs,” said an engineer with a smaller midwestern business.
“Meeting FDA regulations for compliance and document controls,” was cited by an R&D person at a large Midwest nutrition products maker.
Two interesting concerns were raised by a research and development executive with a large northeastern company who admitted, “I’m in the science side of pharmaceutical development and, at best, distantly related to the end-user side of the business. That said, my first issue is the leachability of plasticizers, cross-linkers, and other additives into the product. Hopefully new plastics with lower amounts of these materials can be introduced, or plastics with Teflon-like inertness can be developed.”
His second issue concerned the “need to introduce recyclable or biodegradable products. Greater recyclability is probably more suitable for food products than medical [because] handling of medical waste is a problem. For medical packaging biodegradability, even slow [degradation] would be better than most current products that last ‘forever’ in landfills. I appreciate that my requests may, in fact, compete with each other both in terms of functioning chemistry and cost.”
In a future issue, Packaging World survey respondents elaborate on repackaging, validation, and FDA’s 21 Code of Federal Regulations Part 11 guidelines.
For survey methodology, see: packworld.com/go/w071