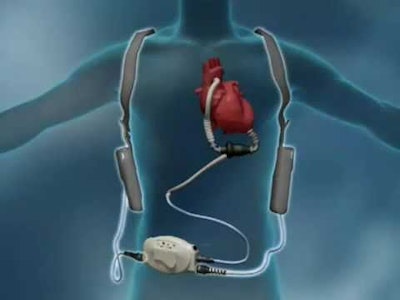
A recent PharmaVoice article reported Abbott Laboratories has issued a massive recall after its HeartMate II blood pumps contribute to 26 patient deaths, and 19 injuries. The HeartMate II is a Left Ventricular Assist Device that pumps a patient’s blood when the heart muscle is too weak. Abbott acquired the medical device earlier in 2017 in a $25 billion deal with St. Jude Medical.
Although the incident is classified as a recall, Abbott isn’t actually requesting the return of any products. Rather, they are asking physicians to make sure their patients complete the exchange in a clinical setting. This isn’t Abbott’s first encounter with heart pump issues; in February, the use of their HeartMate PHP was halted after multiple malfunctions and a patient death.


























