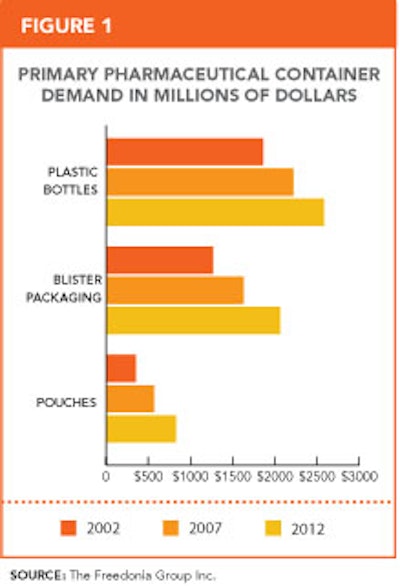
So says a new report available from Freedonia Market Research (www.freedoniagroup.com). Regulations and standards that address issues such as barrier protection, infection control, patient drug compliance, drug-dispensing errors, and drug counterfeiting will drive the gains, the report finds. An increased focus on these issues will result in strong growth for high, value-added container accessories, especially parenteral vials and flip-top closures, plastic dispensing bottles and closures, prefillable inhalers, prefillable syringes, and related closures, rubber, and elastomeric parenteral stoppers, track-and-trace and authentication labels, and unit-dose pouches.





























