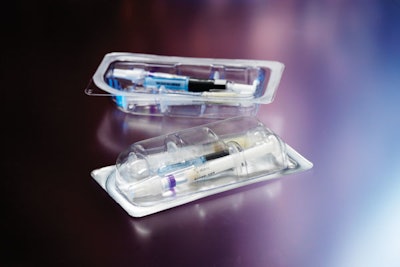
As specialists in formulating, filling, and packaging of pharmaceutical products, Tolmar, Inc. of Fort Collins, CO, has been packaging pre-filled syringes for more than a decade. That’s nothing new, though pre-filled syringes are something of a niche market.
However, using barrier thermoforming material for packaging of pre-filled syringes into trays is new. According to Lori Fischer, Tolmar’s engineering manager, “The contents of the polypropylene syringes can be damaged by exposure to air or moisture, which is why we need barrier packaging in the first place. We had been using a foil pouch with a tear notch, but it was cumbersome. You had to shake the contents out. Also, once packaged, you could not see inside to do a check of the contents.”
Enter Klöckner Pentaplast (www.kpfilms.com) with its Pentamed® Aclar® polyester barrier material. It’s a duplex structure that sandwiches Aclar, a polymer that acts as a moisture and oxygen barrier, between two layers of Klöckner Pentaplast’s high-quality medical PETG. Its use in pharmaceutical packaging is quite common. But it’s new to the medical device field. Tolmar also installed a Model R230 thermoform/seal machine from Multivac (www.multivac.com) when it switched to the new Klockner Pentaplast material. The Multivac, which operates in a Class 100,000 clean room, uses plug assist and vacuum to form trays to a depth of 3⁄4 inch.
“Going from pouches to tray packs using Klöckner Pentaplast’s film has resulted in at least a one-third decrease in costs,” says Fischer. Less material, less costly material, less labor, and a streamlined packaging process combine to produce these savings.
To keep tooling costs to a minimum as the project unfolds and to simplify the FDA approval process, Tolmar opted for a one-size-fits-all, flat-bottomed tray. In Figure A, these trays are being formed four-across. While syringes and needles can be placed directly into these flat-bottomed trays, Figure A illustrates Tolmar’s use of a PVC insert that was requested by the Marketing Department at Tolmar. This insert tray holds and displays the three individual components in a slightly more elegant manner than if they were loose in the Aclar tray. Soon, Tolmar will form these more complex shapes from Aclar and do away with the tray-in-a-tray approach. More on this later.
One of the PP syringes in Figure A contains an active ingredient and the other a carrier. The syringe holding the active ingredient is filled in Tolmar’s aseptic suite at the Fort Collins plant.
Before the switch to the new thermoformed tray, Tolmar operators would insert one syringe into a foil pouch and seal it. Subsequently, they’d put the pouched syringe into a second foil pouch along with the second syringe. The entire package assembly was done by hand, which also introduced a greater risk for repetitive-motion injuries.
Faster and more efficient
Now, says Fischer, “Production is so much faster and more efficient. The Multivac thermoforms 4 or 5 trays in a continuous line. A lot fewer people are needed. “ She adds that it was Multivac who recommended Klöckner Pentaplast as the material supplier.
The other key material component in the new packaging format is the lidding material. A foil/polyester structure from Constantia Hueck (www.constantia-hueck.com), it’s heat-sealed over the formed trays after operators place the syringes and needles inside. Individual trays are cut free from their thermoformed sheet and discharged for insertion into a folding carton.
To administer the drugs, healthcare personnel peel the lidding material from the tray and remove both syringe caps from the syringes. The two syringes, one with a threaded male leur connector and the other a threaded female leur connector, are coupled so that their contents can be mixed. Once mixing of active ingredient and carrier is complete, the combined components are kept in the syringe with the male leur connector. The syringes are uncoupled, and the needle is removed from its container and is screwed onto the male leur connector. The contents are now ready for injection into the patient.
Worth noting is that the carrier component contained in one syringe is activated by water. That’s one reason why, says Fischer, the Pentamed Aclar material was chosen.
“Once the carrier substance hits water, it breaks down,” says Fischer. “That’s why moisture protection is so important, and that’s what led us to Pentamed Aclar for its outstanding barrier properties. It has the same moisture-barrier equivalent as the foil pouches that have been replaced.”
Currently, Tolmar is using the new tray design for two different products: Atridox® and Eligard®. Atridox is a locally delivered antibiotic gel used by dentists to manage adult periodontitis. Tolmar manufactures and markets Atridox on its own. Eligard® is a hormonal therapy for prostate cancer. It is manufactured and packaged by Tolmar for another pharmaceutical firm.
On to the next phase
As Tolmar moves ahead with this ambitious packaging transition, for many syringes it will move away from flat-bottomed thermoforms and will instead thermoform complex contoured trays like the one shown in Figure B, and it will form them from barrier Aclar sheet so that the tray-in-a-tray approach won’t be necessary. To get to this stage, Tolmar will continue taking advantage of BlisterPro™, a value-added service Klöckner Pentaplast offers its customers. It’s a modeling service that evaluates packaging with a combination of 3-D, form/seal machine experiments, and Finite Element Analysis (FEA). According to BlisterPro architect Steve Warakomski, technical manager for pharmaceutical films, “We run experimental matches based on assumptions and then recommend the best film for the application. BlisterPro™ allows us to frame the right question to arrive at better film-choice decisions according to data that’s interpretable, realistic, and makes common sense.”
Often the main questions center on the thinnest area of the design part, which is where trouble spots for stress may occur. With the typical pharmaceutical blister pack, such questions are easier to answer, partly because it’s been done a number of times on other pharma products. But as the first medical device BlisterPro™ project, the weak spots in Tolmar’s tray design were not so readily apparent. So Warakomski set to work pitting experiment vs. prediction to recommend a Pentamed® ACLAR® film with confidence.
Warakomski says the BlisterPro modeling service is especially helpful when it comes to designing the angle of Tolmar’s tray wall. “Tolmar required well defined corners and asked what film we could recommend,” he says. “With the tray design, there is a deeper draw that makes formation around the corners more complex. Also, connecting the right and left walls across a large space is more difficult. The film has to form into the corners without blowing a hole or under-forming. There were an assortment of thicknesses and films that we might have suggested for the corner radius. But it helps to narrow the choices down by process of elimination with BlisterPro.”
To run BlisterPro, Warakomski uses a variety of methods, including proprietary software that he developed. Into the Tolmar equation he loaded
• CAD drawings of the Multivac tooling design, which Multivac supplied
• the forming web being tested • properties that Tolmar specified, such as gamma sterilization capability, and
• the rheological properties of the forming material, i.e., the manner in which the material flows when softened.
“The corners were the thinnest, as expected,” says Warakomski. “That was the weak spot in pressure forming using air to make the part according to the contour plot.” (The contour plot in Figure C shows the expected thickness distribution using a mesh configuration.)
Tolmar had requested a minimum thickness of 3 mil. So Warakomski specified a 15-mil base film in a 15-mil PETG/3-mil Aclar structure to avoid thinning in the corners to a value less than Tolmar’s 3-mil requirement. He might have recommended a 12-mil PETG/3-mil Aclar, but he felt this thinner film might not consistently meet Tolmar’s 3-mil minimum requirement.
To be certain of his recommendation, Warakomski tested 30 formed trays under different conditions on a bell curve for the 3-mil requirement. “We started to see problems when we used 12-mil PETG,” Warakomski explains. “So even though thinner film would provide a better yield and less expense, I felt my recommendation saved problems in the end. We make our Pentamed Aclar film to order. So if the wrong formula is requested, it cuts into production schedules and causes delays, not to mention incurring unrecoverable cost.”
Downgauge later if need be
Warakomski goes on to explain that Tolmar can always take costs out later by doing such things as cutting out depth or changing wall angle. “We sell our customers what they need for their FDA application,” he adds. “Better safe than sorry.”
Tolmar’s Fischer concurs. “We don’t have to do additional clinical trials on either product,” she points out, “but we do have to make an amendment to our package design and back it up with a study of accelerated stability conditions. We simulate the two-year shelf life of the drug with temperatures to 40 degrees centigrade, and we conduct ship-testing as if the package were being transported on a truck. We are converting to these new packages for our products distributed in Europe. And because of different regulatory standards in each country, it has taken two years to get both products into trays.” Eligard® goes right to urologists’ offices or to clinics where it is administered. Atridox is sold directly to dentists and dental clinics. “In fact,” Fischer says, “this is good timing now that we have a pretty package. We are changing from foil pouches to thermoformed trays just as we are building up a sales and marketing force to promote Atridox.”
“I’m pleased to see that the tray is forming as cleanly as it is,” says Warakomski. “The film we recommended has uniform formation in the bottom large cavity and good thickness in the cross sections. It didn’t stretch apart.” The BlisterPro technology, he reiterates, helped greatly in producing this outcome.
He also expects BlisterPro techniques to improve and become more comprehensive with progressive opportunities for customers. He plans to build in more variables such as film characteristics and seal behavior. At one time, the mold and the technology needed to be tested and adjusted. Now BlisterPro is more straightforward with testing between the film and its application.
As for its application at Tolmar, it appears to be a winner. “Kudos to Klöckner Pentaplast for getting us through the FDA amendment process,” says Fischer. “We now have a clean-looking, polished package that is easy to use and cuts both labor and material costs.”