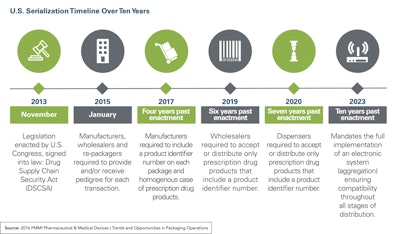
Serialization and aggregation catalyze a paradigm shift for the entire healthcare industry, according to the recent survey, “2016 PMMI Pharmaceutical & Medical Devices: Trends and Opportunities in Packaging Operations.”
Nearly half of survey participants were more than 50% compliant-ready to meet advancing serialization and aggregation deadlines. Of the companies interviewed that are required to serialize their products, 52% have already adopted aggregation.
There are several hurdles to serialization implementation, according to the survey, which points out the following:
• Cost. To implement serialization at one manufacturing site can cost anywhere from $1 to $4 million. The costs are driving smaller companies out of business.
• Internal infrastructure. Database integration and communication infrastructure optimization to determine the best method to capture, manage, and transmit data.
• Validation and documentation. Defining rules and what’s required to meet all regulations.
• Time. In small test runs serialization works, but ramping up to full-scale production takes time to work through all the challenges and exceptions.
• Equipment purchases. Difficulty acquiring and procuring equipment when there are a limited number of suppliers; some equipment purchased has an ROI of 30 years. Machine delivery times can be as high as two years.
One pharmaceutical sterilization expert responding to the survey noted,
“Pharma companies will look for ways to recover costs by improving manufacturing, packaging, and handling processes—packaging changes are mandatory because all serialization information can’t fit on some products.”
A maintenance manager with a generic pharmaceutical company simply stated, “Serialization directly affects OEE negatively.”
Contract service providers could provide help for firms needing assistance with serialization. The PMMI study noted the following contract services advantages:
• Mitigates the purchase of complex equipment and overall costs of implementation.
• Specifications and serialized numbers typically provided by brand owner for contractors to use.
• Experienced in complying with different country regulations.
• Able to integrate a variety of track-and-trace systems.
A leading pharmaceutical expert responding to the survey added this insight: “Serialization for prescription drugs is the foremost area where contract packagers are looking to expand capabilities since the Drug Quality and Security Act (DQSA), 2013. A modular machine concept is needed in order to be compatible with existing lines equipped with matching software.”


























