The U.S. Food and Drug Administration is likely to reject Princeton, NJ-based Bristol-Myers Squibb’s bid to co-package its cholesterol-lowering drug Pravachol with aspirin, both serving as a means of preventing secondary coronary disease. In doing so, the FDA would be listening to its Cardiovascular and Renal Drugs Advisory Committee, which on January 18, 2002, voiced a number of reservations about the Pravachol/ aspirin combo package.
According to one FDA source, a key advisory committee objection—but hardly the only one—was that aspirin should not be co-packaged with anything, whether Pravachol or any other prescription drug.
Much of the discussion of the advisory committee, composed of cardiologists, pathophysiologists, biometricians, and persons of other medical disciplines, was in language that only a lab rat could understand. But clear policy concerns did emerge from the medical arcania.
First and foremost is the absence of any coherent FDA policy on co-packaging of drugs or any other products—toothpaste and toothbrushes, for example. The few pharmaceutical combinations approved in recent years have slid through the FDA on an ad hoc basis, not measured against any standards.
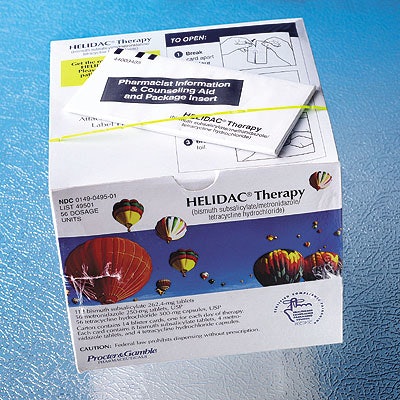
Perhaps the best known is the Helidac therapy package for ulcer sufferers sold by Prometheus Laboratories, Inc., of San Diego, CA. Each package includes 14 blister cards, one to be used per day. Each of the individual cards is subdivided into four tear-open parts, containing four capsules: one of metronidazole, a second of tetracycline hydrochloride, both antibiotics, and two of bismuth subsalicylate, better known as Pepto Bismol.
Another combination, this time from Andrx Corp., Davie, FL, is Embrex 600, a prenatal vitamin co-packaged with a chewable calcium tablet. It has been on the market for about a year.
Crystal Rice, an FDA spokeswoman, says the agency has no comprehensive list of co-package approvals. Those ad hoc approvals, and the continuing submission of applications, such as Bristol Myers’s, for other co-packaging arrangements, led to the establishment of a co-packaging subcommittee within the FDA’s Medical Policy Coordinating Committee about two years ago.
Suggesting policy
Wiley Chambers, the FDA official who chairs the co-packaging subcommittee, says his group put together a document setting out suggested FDA policy for co-packaging. That document was sent to the FDA’s regulatory policy group early in 2001. That group would have to approve the document before it can be published in the Federal Register, an event that will set off a public comment period.
The FDA’s cardiovascular and renal drugs advisory committee certainly could have put such a policy to good use in its consideration of the Bristol-Myers application. Fred Fiedorek, a Bristol-Myers executive who made the company’s presentation to the FDA advisory committee, underlined the compliance rationale for his company’s application.
In Bristol-Myers case, Fiedorek argues that the Pravachol/aspirin co-package would make it easier for physicians and patients to comply with the American Heart Assn.’s 1995 guidelines on risk reduction in patients at risk for coronary heart disease. Those guidelines call for between 80 and 325 mg of aspirin a day and a 40-mg dose of a cholesterol-lowering drug called a statin, of which Pravachol is one example.
The everyday problem, according to Fiedorek, is that patients go to the drugstore and buy aspirin substitutes such as Tylenol that do not have the same prophylactic effect as aspirin.
Some drug guides
There are clinical guidelines for many diseases, such as diabetes and hypertension, that call for more than one drug to be taken, making the likelihood of future co-packaging applications a good bet.
“We are looking at combinations of other drugs just like Bristol-Myers,” says Bruce Cohen, a top packaging executive at GlaxoSmithKline, Philadelphia, PA. GSK already markets a combo package called Advair. “We will take them one at a time.”
“Co-packaging is a coming thing,” says Domenic Cianco, vice president of sales for American Health Packaging, Columbus, OH. His company does the packaging for Embrex 600. “Co-packaging is particularly useful for people who travel in their jobs,” he explains. “The blister cards are marketed for each day of the week and are a lot easier to carry on trips than bottles of pills.”
While co-packaging can make life easier for a patient, it can make it more difficult for a physician to ensure that the patient is receiving the correct dosage of medication. Bristol-Myers proposed making two co-packages available: both include 40- mg tablets of Pravachol; one has an 81-mg dose of aspirin, the other comes with 325 mg of aspirin.
The level of lipids in the bloodstream of patients at risk for coronary heart disease must be checked frequently. “We know there is abundant data that the first dose that patients start on is often the dose that they stay on,” explains Steven Nissen, M.D., vice chairman of the Department of Cardiology at the Cleveland Clinic Foundation and also a member of the FDA advisory committee. He argues that the availability of co-packaged Pravachol and aspirin would make it more difficult for a physician to get a patient to accept a different statin, or perhaps another aspirin level, should the patient’s lipid level dictate that.
Beyond drugs
A co-packaging policy—both the absence of one and the establishment of one—has implications for products beyond drugs. The FDA’s Chambers points out that toothbrushes are sometimes packaged with toothpastes. The FDA has allowed this because it makes sense—not because it squares with any formal policy. “We don’t want any new policy to exclude current co-packages that make sense,” Chambers states.
Such a policy would be welcomed, and not only by drug companies. Peter Mayberry, executive director of the Healthcare Compliance Packaging Council, tells the story of a Boston gynecologist who developed his own combination packaging for calcium and a hormone replacement therapy. These were paired together for women in danger of losing bone density.
The gynecologist approached the FDA with his package but was told he would have to conduct clinical trials to get approval for his packaging. That, of course, was not true. Bristol-Myers did not conduct clinical trials, nor was it required to, for its application for co-packaging of Pravachol and aspirin.
However after hearing FDA’s mistaken counsel, the Boston gynecologist just shook his head and dropped the idea.