My 20-month-old grandson Dustin is a bit on the small side. When my daughter Brittany takes him to the family practice physician, his percentile ranking in height and weight is based on a comparison to others of his age. Yet when Dustin is sized up at our local Women’s Infants-Children food and nutrition office, they determine his percentile growth ranking not based on a comparison with others of his age, but with how his height matches with his weight, and vice-versa. That makes a difference.
When it comes to using weight measurements versus age in determining dosing for children’s medications, including acetaminophen, apparently a U.S. Food and Drug Administration advisory panel is tipping the scales in favor of weight. Specifically, the panel is recommending, “dosing for children’s acetaminophen be based primarily on the child’s weight rather than age.” That’s according to a May 19 Wall Street Journal Health blog, “Panel calls for label changes on acetaminophen products.”
The article says, “Johnson & Johnson's McNeil Consumer Health Care, which makes Tylenol, has asked the FDA to formally amend rules governing over-the-counter products to allow for weight-based dosing and to allow the instructions on children's packages to include infant instructions. The FDA is expected to adopt many of the panel's recommendations as part of likely changes to over-the-counter drug regulations for dosing and packaging.”
Meanwhile, on its Tylenol Web site, the company says, “Recently, a panel of experts convened by FDA voted to recommend enhanced label directions for infants’ and children’s acetaminophen products. The panel unanimously recommended adding dosing directions for children ages 6 to 23 months to the package label of infant’s acetaminophen products. The makers of Children’s TYLENOL® requested this label enhancement and are pleased that it was supported by the expert panel. We believe including dosing directions for children ages 6 to 23 months directly on the product label will further assist parents and caregivers in using our products appropriately.”
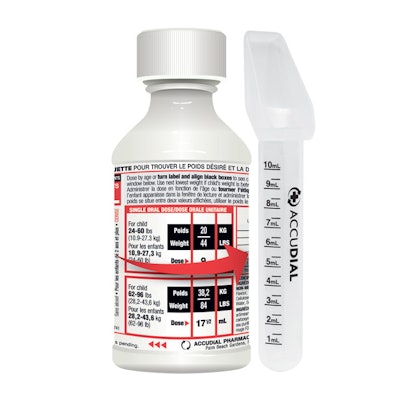
Shown above is package labeling that focuses on dosage based on weight, which comes from AccuDial Pharmaceutical, Inc., which won the Edison Best New Product Award in the Consumer Packaged Goods Category for AccuDial®, a line of pediatric medications that features a two-part, patented label system in which the consumer rotates the top label to display the child’s weight, with dosing information revealed on an inner label to allow the caregiver to provide an exact medicine dose with a calibrated dosing device included in the package. According to the product’s Web site, “The weight of children in the same age group can vary greatly, and they should not always be given the same amount of medicine. According to the American Medical Association, 72% of children are dosed inaccurately with OTC medications.”