How to prevent caregivers and healthcare workers from accidental exposure to toxic cancer drugs, even as more and more of these drug regimens are being administered at home? It could be as simple as preventing a vial from breaking.
Vials of expensive cancer drugs can be highly toxic, and when a glass vial shatters and the contents are scattered, there are dire health consequences for those who package, transport, administer and discard the drugs. Not to mention costly cleanup and decontamination procedures in the event of breakage.
A 2011 study examined the risk to nurses treating cancer patients being exposed to chemotherapy drugs and their toxic effects. The results showed that nearly 17% of nurses working in centers where outpatient chemotherapy infusions are administered reported being exposed on their skin or eyes to the drugs. The researchers surveyed 1,339 oncology nurses working in Michigan in outpatient settings. About 84% of chemotherapy is delivered in such settings, the researchers said.
Unintentional chemotherapy exposure can affect the nervous system, impair the reproductive system and bring an increased risk of developing blood cancers in the future, the researchers said. These exposures can be as dangerous to a nurse's health as an accidental needle stick.
Safety guidelines, such as recommendations for using gowns, gloves and other protective gear when handling chemotherapy drugs, have been issued by organizations such as the National Institute for Occupational Safety and Health, but these guidelines are not yet mandatory, the researchers said.
Protecting the vial
The following guidelines regarding packaging and segregation of cancer drugs were recently recommended by an international pharmacy panel:
• Effective packaging and segregation techniques should be used to avoid contamination prior to distribution.
• Packaging should clearly state whether segregation techniques have been used so that individuals unpacking the medications can take additional precautions if necessary.
• Packaging material should be durable, able to contain any accidental leakage during handling and transport, and be tamper-proof.
• Package label should indicate that the agent is cytotoxic.
• Distributors should ensure that the labeling on the packaging is intact and that oral cytotoxic agents are stored and transported separately from non-cytotoxic agents.
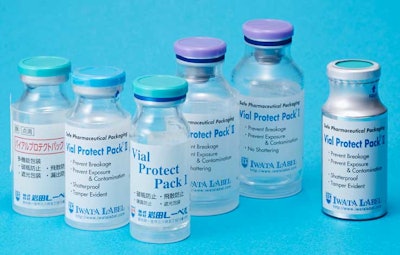
In his Pharma EXPO Innovation Stage presentation, “Risk in the Workplace—Preventing Shattered Vials and Protecting Handlers of Highly Toxic Drugs,” Chris Ostein of Iwata Label presented two of the company’s designs to minimize the risk of breakage.
Vial Protect Pack I (VPP I) uses a PET plate attached to the bottom of the vial, followed by a PE “shrink-tack” label for a cushioning effect.
• In the Vial Protect Pack I design, a protector is joined to the base of the vial by one shrink-tack label with the printed display, with the goal of preventing bottle breakage.
• The shrink-tack label covers the entire vial from the aluminum cap to the base and prevents spillage of the contents.
• Light-resistant film wrapping from the aluminum cap to the base protects the formulation.
• After drawing the medical solution, part of the label can be removed and attached to the syringe.
Vial Protect Pack II (VPP II) goes even further and features a protector PET cup to enclose the glass vial, followed by the PE shrink-tack label.
• In case the vial breaks, the shrink-wrap film prevents shattering and splash.
• Shrink-tack label holds the vial and the protector firmly with adhesive; therefore the vial does not slip out.
• There is no special handling required because the Vial Protect Pack II fits the vial and does not affect the shape or the size of the vial.
• Customer’s existing shrink-tack labeler can be used.
Extensive drop testing from various heights proved that the two methods were a vast improvement over unprotected vials; in vertical drop tests from heights of 1, 1.5 and 2 meters, the VPP 1 package eliminated breakage completely when tested against a standard vial. And even if breakage of the glass vial occurs in shipping or in a clinical setting, the shrink wrap keeps the contents contained.
The Iwata packaging allows the drug packager to, in many instances, reduce the amount of secondary packaging, such as paperboard sleeve dividers and spacers, needed for the vials. Iwata manufactures both the labels and the labeling machinery in Japan.