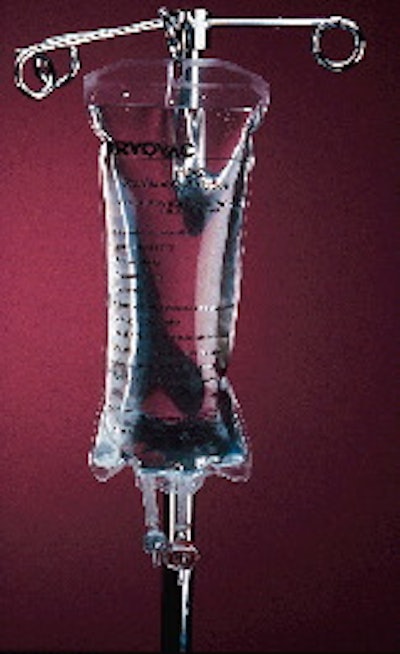
Packages representing significant technical accomplishments in the medical or surgical fields are always among the winners in the Flexible Packaging Assn.'s annual awards competition. This year is no exception. Medical/surgical winners include a multilayer bag for intravenous solutions, a pressure-formed foil-based package for a catheter cap and a flexible package that replaces a rigid tray for surgical devices. (For coverage of other FPA award winners, please see p. 30 and p. 78.) The rigid tray replacement so impressed FPA judges with its environmental benefits, they honored it with the fifth annual Green Globe Award for environmental achievement. Winning the prestigious award was Rexam Medical Packaging (Vernon Hills, IL) for a pouch produced for Cincinnati-based Ethicon Endo-Surgery, Inc., a division of Johnson & Johnson. Ethicon began using the pouch last October for about 30 different devices, including its Endopath® surgical trocar and Proximate® skin staple extractor (1). Ethicon ships these devices worldwide, either through distributors or directly to hospitals. The devices are sold in a thermoformed/filled/sealed pack, in 3"x6" or 3"x10 1/2" configurations. Each unit is then packed in a paperboard shelf carton. Cartons go in corrugated shippers that are sterilized by radiation before shipping. The forming web for the new package is Integra® Form C film, supplied by Rexam. The 12-mil, three-layer coextrusion consists of ethylene vinyl acetate/ionomer/EVA. Lidstock is a coated 1073B Tyvek® from DuPont (Wilmington, DE). The two materials are united on two new customized Mahaffy & Harder Engineering (Fairfield, NJ) tf/f/s machines. During the process, a male plug descends into the heated web of film. Below the film is a flat aluminum plate. Vacuum is drawn from above the film, pulling the film up and around the male plug, which is manufactured to precision tolerances for each of Ethicon's products. The film takes on the shape of the male tool. By using these custom male plugs, Ethicon can perform changeovers much more quickly than it could if female cavities had to be exchanged. After forming, the products are placed in the cavities and sealing follows. This is done four-up across the 12 1/4"-wide film web. Ethicon had used a 35-mil premade rigid tray before making the change to the film structure. "We wanted to reduce the amount of packaging waste," says Peter Herrmann, a staff engineer for Ethicon. Herrmann, who works at the company's Albuquerque, NM, plant where packaging is done, confirms Rexam's claim that the switch to the flexible structure saves approximately 80% by weight, with as much as 350ꯠ lb/yr of material that no longer needs to be landfilled. "We wanted to reduce our costs, and provide packaging that was more appropriate for our products. The impact on our company from the switch has been phenomenal," Herrmann says. "There are significant savings both in material and labor costs compared to using the trays." With trays, operators hand-filled the surgical devices, then placed die-cut lids on top, using a shuttle-type sealing machine to seal lids to the filled tray. By using flexible film rather than rigid trays, Ethicon's customers also benefit. "Hospitals often have to discard this packaging as medical waste," Herrmann tells PW. "That's very expensive for them to handle. I've heard of hospitals spending in the hundreds of thousands of dollars per year for medical waste disposal. With film the reduction in weight provides them with a significant financial benefit. "We've received a very positive customer feedback to this package," he adds, "though it would be difficult for us to attribute additional sales to the package change at this time." Beyond the obvious cost savings, the switch to the film is a source of pride to Herrmann. "If you look at the packaging for other industries, particularly for consumer products, it has changed quite a bit. Soda bottles, for example, have been lightweighted to use less material. "However, in the area of medical devices," he contends, "packaging has been a little staid. If you took a package from 10 years ago and compared it to one today, you'd see little or no difference. It's a little unusual for surgical devices like these to be packaged in a flexible film material. It gives our products a point of differentiation." Sterilizable medical film Developed as an alternative to intravenous (IV) solution bags made of polyvinyl chloride, Cryovac's (Duncan, SC) new M312 sterilizable medical film (2) was recognized by FPA judges since it's thinner than some competing materials, lacks plasticizers that interfere with product purity, and resists sub-freezing temperatures. The multilayer coextrusion has about one-third the moisture permeability at half the thickness of 15-mil PVC, a standard IV bag material, according to Cryovac. The barrier allows the material to compete with glass for certain applications, says the company. The heat-sealable film also retains its high clarity after autoclave sterilization. The film was first commercialized in early 1996 by Verona, Italy-based S.I.F.R.A. Intl., one of that country's largest pharmaceutical companies. It replaced 350-micron (13.8-mil) PVC for certain of that company's IV solutions, according to Basile Nahas, S.I.F.R.A.'s president. While M312 is more expensive than the PVC film that S.I.F.R.A. had been extruding in-house, according to Nahas, it reportedly allows the pharmaceutical company to use the material in markets where PVC is considered undesirable for environmental or other reasons. Cryovac acknowledges that M312 may cost more than PVC film that's extruded in-house. But the converter says that most pharmaceutical companies buy PVC in rollstock or in pouch form, rather than extruding it in-house, so they should find M312 competitive compared to PVC. Cryovac coextrudes the film in tubular form and maintains Class 100 filtered air on the inside of the tube to minimize particulate contamination of product-contact areas. The supplier declines to identify materials, except to say that the outer layer is polyester and the others are polyolefin-based. At S.I.F.R.A., the bags are formed, filled and heat-sealed on custom equipment developed by S.I.F.R.A. itself, though Cryovac says commercial equipment is available. Baxter's MiniCap The majority of people with kidney disease rely on dialysis, the process of cleansing toxins from the blood to keep them alive after their kidneys have failed. Many patients conduct their own dialysis at home through peritoneal dialysis, which uses the body's peritoneum (or abdominal cavity) as a filter while it infuses and drains dialysis solution. The dialysis solution is infused into and drained through a catheter in the patient's abdominal cavity. Between dialysis treatments, patients must cap off their catheter. Baxter Healthcare, a division of Deerfield, IL-based Baxter Intl., makes a plastic threaded "MiniCap, packed with an iodine-laden sponge" for just this purpose, and its redesigned package (3) is among this year's FPA winners. Baxter's accomplishment is impressive, involving what it calls a "lights-out" Tiromat system from TLF Medical Packaging (Avon, MA) that performs not only primary packaging, on-line printing, robotic cartoning, and print-on-demand carton labeling, but also assembles the MiniCap itself. The Tetra Laval system, installed at Baxter's Cleveland, MS, facility, produces the primary pack on high-speed horizontal pressure form/fill/seal equipment fed by two foil laminations supplied by Rollprint (Addison, IL). Because of the nature of kidney disease, some patients have limited dexterity. The new pack requires less force to peel open and is easier for these patients. Once open, the formed bottom web becomes a tray from which the patient can easily grasp the cap in such a way that touch contamination of the sterile product is minimized. The key behind the new package was Rollprint's development of two new heat-sealable foil laminations that offer peelable seals and high barriers to moisture and oxygen. Especially critical are the two sealant layers. Not only must they provide seal integrity and peelability, they must also provide chemical resistance to iodine, a chemical notoriously hard-to-hold in a foil lamination. The bottom web is also significant because it is cold-formed to a depth of about 0.5" without loss of barrier or chemical resistance properties. The new MiniCap package delivers benefits to Baxter as well as to the patients who use it: * It's about 40% smaller than premade pouches currently in use. * The small size lets Baxter reduce the size of its corrugated shippers and gain added efficiency in warehousing and distribution. * It reduces packaging waste by about a third.