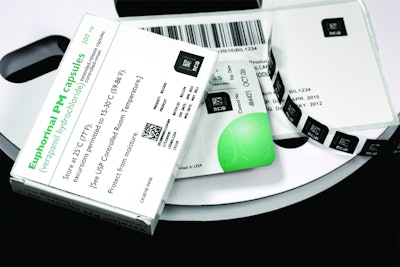
The system integrates into existing packaging operations as unit-level labels on blister packs, secondary packaging, or tamper-evident closure labels; nonClonableID addresses the need for instant authentication, secure track-and-trace, and e-pedigree in a scalable, cost-effective manner.
It employs a unique materials-based fingerprint for each product that is impossible to copy. The fingerprint cannot be duplicated even by Bilcare itself. The nonClonableID achieves this level of security through a system of randomly placed micro- and nano-particles that make up a one-of-a-kind authentication tag. When combined with the tags’ real-time verification capabilities, the result is a foolproof authentication process whose levels of assurance far exceed most existing security technologies, according to the company.
These materials-based fingerprints are applied as tamper-evident labels on product packaging. Authentication is available in just seconds through a commercially available reader that checks a scanned fingerprint against an online database; the information can even be sent to individual smartphones. nonClonableID also can be custom-designed to address product-specific integrity challenges.
The technology behind nonClonableID also can assist with patient compliance by ensuring proper dispensation of medication and compliance with medication regimen via real-time monitoring by the healthcare provider/pharmacist.

























